Protocol: The Lacunar Intervention Trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease
- PMID: 33072884
- PMCID: PMC7538764
- DOI: 10.1177/2396987320920110
Protocol: The Lacunar Intervention Trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease
Abstract
Background: Small vessel disease causes a quarter of ischaemic strokes (lacunar subtype), up to 45% of dementia either as vascular or mixed types, cognitive impairment and physical frailty. However, there is no specific treatment to prevent progression of small vessel disease.
Aim: We designed the LACunar Intervention Trial-2 (LACI-2) to test feasibility of a large trial testing cilostazol and/or isosorbide mononitrate (ISMN) by demonstrating adequate participant recruitment and retention in follow-up, drug tolerability, safety and confirm outcome event rates required to power a phase 3 trial.
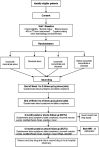
Methods and design: LACI-2 is an investigator-initiated, prospective randomised open label blinded endpoint (PROBE) trial aiming to recruit 400 patients with prior lacunar syndrome due to a small subcortical infarct. We randomise participants to cilostazol v no cilostazol and ISMN or no ISMN, minimising on key prognostic factors. All patients receive guideline-based best medical therapy. Patients commence trial drug at low dose, increment to full dose over 2-4 weeks, continuing on full dose for a year. We follow-up participants to one year for symptoms, tablet compliance, safety, recurrent vascular events, cognition and functional outcomes, Trails B and brain MRI. LACI-2 is registered ISRCTN 14911850, EudraCT 2016-002277-35.Trial outcome: Primary outcome is feasibility of recruitment and compliance; secondary outcomes include safety (cerebral or systemic bleeding, falls, death), efficacy (recurrent cerebral and cardiac vascular events, cognition on TICS, Trails B) and tolerability.
Summary: LACI-2 will determine feasibility, tolerability and provide outcome rates to power a large phase 3 trial to prevent progression of cerebral small vessel disease.
Keywords: Lacunar stroke; cilostazol; isosorbide mononitrate; randomised clinical trial; small vessel disease.
© European Stroke Organisation 2020.
Figures
References
-
- World Health Organisation. The top 10 causes of death, www.who.int/mediacentre/factsheets/fs310/en/. (2014, accessed 29 January 2015).
-
- Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18: 684–696. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

