Aspects of Newborn Screening in Isovaleric Acidemia
- PMID: 33072933
- PMCID: PMC7548899
- DOI: 10.3390/ijns4010007
Aspects of Newborn Screening in Isovaleric Acidemia
Abstract
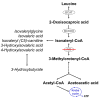
Isovaleric acidemia (IVA), an inborn error of leucine catabolism, is caused by mutations in the isovaleryl-CoA dehydrogenase (IVD) gene, resulting in the accumulation of derivatives of isovaleryl-CoA including isovaleryl (C5)-carnitine, the marker metabolite used for newborn screening (NBS). The inclusion of IVA in NBS programs in many countries has broadened knowledge of the variability of the condition, whereas prior to NBS, two distinct clinical phenotypes were known, an "acute neonatal" and a "chronic intermittent" form. An additional biochemically mild and potentially asymptomatic form of IVA and its association with a common missense mutation, c.932C>T (p.A282V), was discovered in subjects identified through NBS. Deficiency of short/branched chain specific acyl-CoA dehydrogenase (2-methylbutyryl-CoA dehydrogenase), a defect of isoleucine degradation whose clinical significance remains unclear, also results in elevated C5-carnitine, and may therefore be detected by NBS for IVA. Treatment strategies for the long-term management of symptomatic IVA comprise the prevention of catabolism, dietary restriction of natural protein or leucine intake, and supplementation with l-carnitine and/or l-glycine. Recommendations on how to counsel and manage individuals with the mild phenotype detected by NBS are required.
Keywords: blood C5-carnitine; isovaleric acidemia; mild phenotype; newborn screening; short/branched chain specific acyl-CoA dehydrogenase.
© 2018 by the authors.
Conflict of interest statement
Conflicts of InterestThe authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

