Duchenne Muscular Dystrophy Newborn Screening: Evaluation of a New GSP® Neonatal Creatine Kinase-MM Kit in a US and Danish Population
- PMID: 33072986
- PMCID: PMC7510235
- DOI: 10.3390/ijns5030027
Duchenne Muscular Dystrophy Newborn Screening: Evaluation of a New GSP® Neonatal Creatine Kinase-MM Kit in a US and Danish Population
Abstract
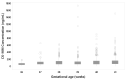
Duchenne muscular dystrophy (DMD/Duchenne) is a progressive X-linked disease and is the most common pediatric-onset form of muscular dystrophy, affecting approximately 1:5000 live male births. DNA testing for mutations in the dystrophin gene confirms the diagnosis of this disorder. This study involves assessment of screening newborns for DMD using an immunoassay for muscle-type (MM) creatine kinase (CK) isoform-the GSP Neonatal CK-MM kit. Comparisons were made with CK activity determination by fluorescence measurement. In addition, the study evaluated the effect of gestational age, age of infant at time of sampling and how stable the CK-MM was over time. This assay discriminates well between normal, unaffected and Duchenne affected populations and is suitable for Duchenne newborn screening.
Keywords: Duchenne muscular dystrophy; creatine kinase; newborn screening.
© 2019 by the authors.
Conflict of interest statement
Conflicts of InterestA.T., L.M., P.M., V.L., T.P., H.P. and T.K. are PerkinElmer employees, the other authors declare no conflict of interest.
Figures




References
-
- Ciafaloni E., Fox D.J., Pandya S., Westfield C.P., Puzhankara S., Romitti P.A., Mathews K.D., Miller T.M., Matthews D.J., Miller L.A., et al. Delayed Diagnosis in Duchenne muscular dystrophy: Data from the Muscular Dystrophy Surveillance, Tracking, Tracking and Research Network (MD STARnet) J. Pediatr. 2009;155:380–385. doi: 10.1016/j.jpeds.2009.02.007. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

