Incorporation of Second-Tier Biomarker Testing Improves the Specificity of Newborn Screening for Mucopolysaccharidosis Type I
- PMID: 33073008
- PMCID: PMC7422968
- DOI: 10.3390/ijns6010010
Incorporation of Second-Tier Biomarker Testing Improves the Specificity of Newborn Screening for Mucopolysaccharidosis Type I
Abstract
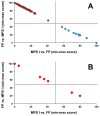
Enzyme-based newborn screening for Mucopolysaccharidosis type I (MPS I) has a high false-positive rate due to the prevalence of pseudodeficiency alleles, often resulting in unnecessary and costly follow up. The glycosaminoglycans (GAGs), dermatan sulfate (DS) and heparan sulfate (HS) are both substrates for α-l-iduronidase (IDUA). These GAGs are elevated in patients with MPS I and have been shown to be promising biomarkers for both primary and second-tier testing. Since February 2016, we have measured DS and HS in 1213 specimens submitted on infants at risk for MPS I based on newborn screening. Molecular correlation was available for 157 of the tested cases. Samples from infants with MPS I confirmed by IDUA molecular analysis all had significantly elevated levels of DS and HS compared to those with confirmed pseudodeficiency and/or heterozygosity. Analysis of our testing population and correlation with molecular results identified few discrepant outcomes and uncovered no evidence of false-negative cases. We have demonstrated that blood spot GAGs analysis accurately discriminates between patients with confirmed MPS I and false-positive cases due to pseudodeficiency or heterozygosity and increases the specificity of newborn screening for MPS I.
Keywords: GAGs; MPS I; biomarkers; newborn screening; postanalytical interpretation; second-tier testing.
© 2020 by the authors.
Conflict of interest statement
Conflicts of InterestThe authors declare no conflict of interest.
Figures

References
-
- Wraith J.E., Clarke L.A., Beck M., Kolodny E.H., Pastores G.M., Muenzer J., Braakman T. Enzyme replacement therapy for mucopolysaccharidosis I: A randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J. Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. - DOI - PubMed
-
- Aldenhoven M., Jones S.A., Bonney D., Borrill R.E., Coussons M., Mercer J., van der Ploeg A.T. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: Results after implementation of international guidelines. Biol. Blood Marrow Transpl. 2015;21:1106–1109. doi: 10.1016/j.bbmt.2015.02.011. - DOI - PubMed
-
- Burton B.K., Charrow J., Hoganson G.E., Waggoner D., Tinkle B., Braddock S.R., Schneider M., Grange D.K., Nash C., Shryock H., et al. Newborn screening for lysosomal storage disorders in illinois: The Initial 15-month experience. J. Pediatr. 2017;190:130–135. doi: 10.1016/j.jpeds.2017.06.048. - DOI - PubMed
LinkOut - more resources
Full Text Sources

