Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences
- PMID: 33073030
- PMCID: PMC7422992
- DOI: 10.3390/ijns6020035
Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences
Abstract
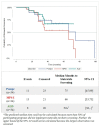
Data were collected from 39 newborn screening (NBS) programs to provide insight into the time and factors required for implementing statewide screening for Pompe, Mucopolysaccharidosis type I (MPS I), adrenoleukodystrophy (ALD), and Spinal Muscular Atrophy (SMA). Newborn screening program readiness to screen statewide for a condition was assessed using four phases: (1) approval to screen; (2) laboratory, follow-up, and information technology capabilities; (3) education; and (4) implementation of statewide newborn screening. Seventeen states (43.6%) reached statewide implementation for at least one new disorder. Those states reported that it took 28 months to implement statewide screening for Pompe and MPS I, 30.5 months for ALD, and 20 months for SMA. Using survival curve analysis to account for states still in progress, the estimated median time to statewide screening increased to 75 months for Pompe and 66 months for MPS I. When looking at how long each readiness component took to complete, laboratory readiness was one of the lengthier processes, taking about 39 months. Collaboration with other NBS programs and hiring were the most frequently mentioned facilitators to implementing newborn screening. Staffing or inability to hire both laboratory and follow-up staff was the most frequently mentioned barrier.
Keywords: Mucopolysaccharidosis type I (MPS I); Pompe; Spinal Muscular Atrophy; X-linked adrenoleukodystrophy (ALD); new conditions; newborn screening; newborn screening readiness.
© 2020 by the authors.
Conflict of interest statement
Conflicts of InterestThe authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Ten great public health achievements—United States, 2001–2010. MMWR Morb. Mortal. Wkly. Rep. 2011;60:619–623. - PubMed
-
- Kemper A.R., Green N.S., Calonge N., Lam W.K., Comeau A.M., Goldenberg A.J., Ojodu J., Prosser L.A., Tanksley S., Bocchini J.A., Jr. Decision-making process for conditions nominated to the recommended uniform screening panel: Statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 2014;16:183–187. doi: 10.1038/gim.2013.98. - DOI - PubMed
-
- Hughes R.I., Fix A. As Advancements In Treatment Drive A Newborn Screening Evolution, Will States And The Federal Government Be Able To Keep Up? [(accessed on 17 April 2020)]; Available online: https://www.healthaffairs.org/do/10.1377/hblog20190816.174106/full/ - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

