Molecular View into the Cyclodextrin Cavity: Structure and Hydration
- PMID: 33073091
- PMCID: PMC7557249
- DOI: 10.1021/acsomega.0c02760
Molecular View into the Cyclodextrin Cavity: Structure and Hydration
Abstract
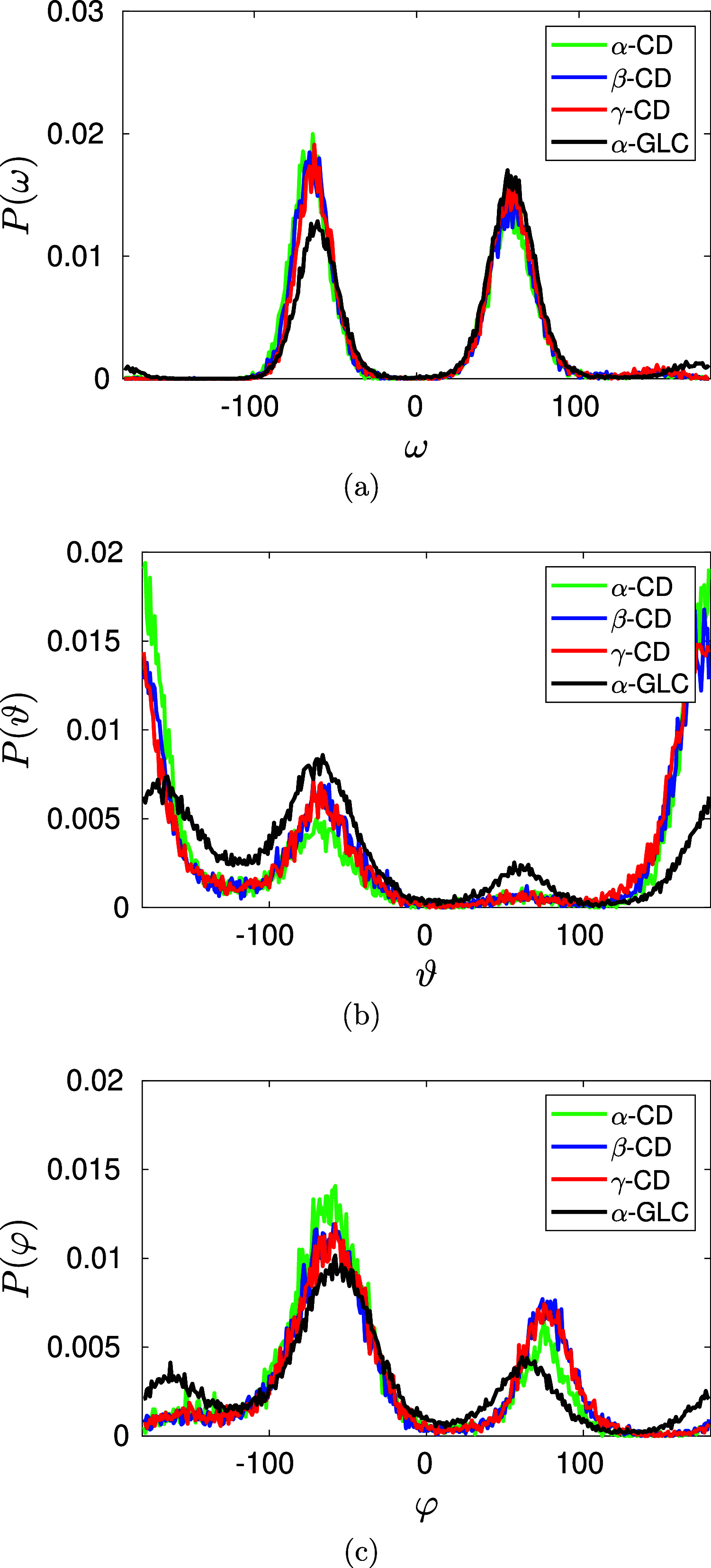
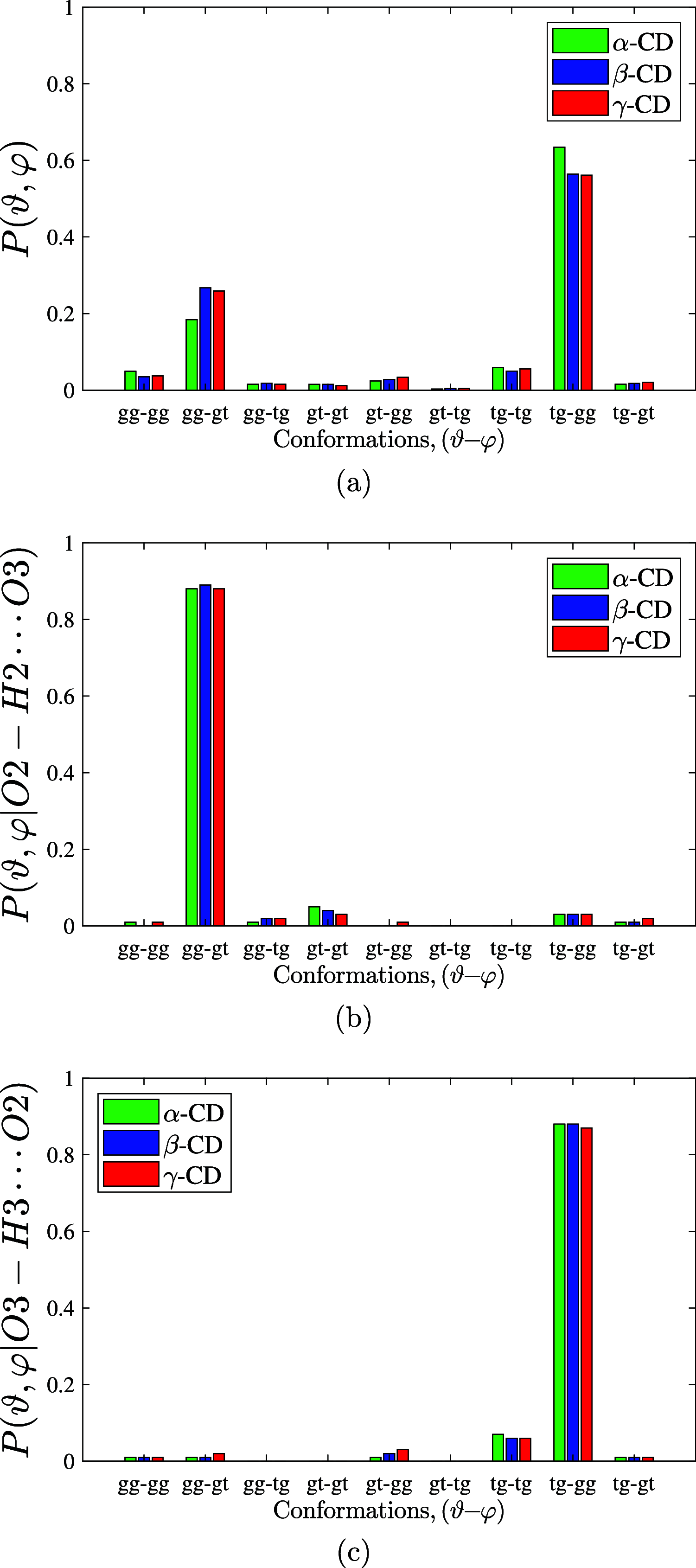
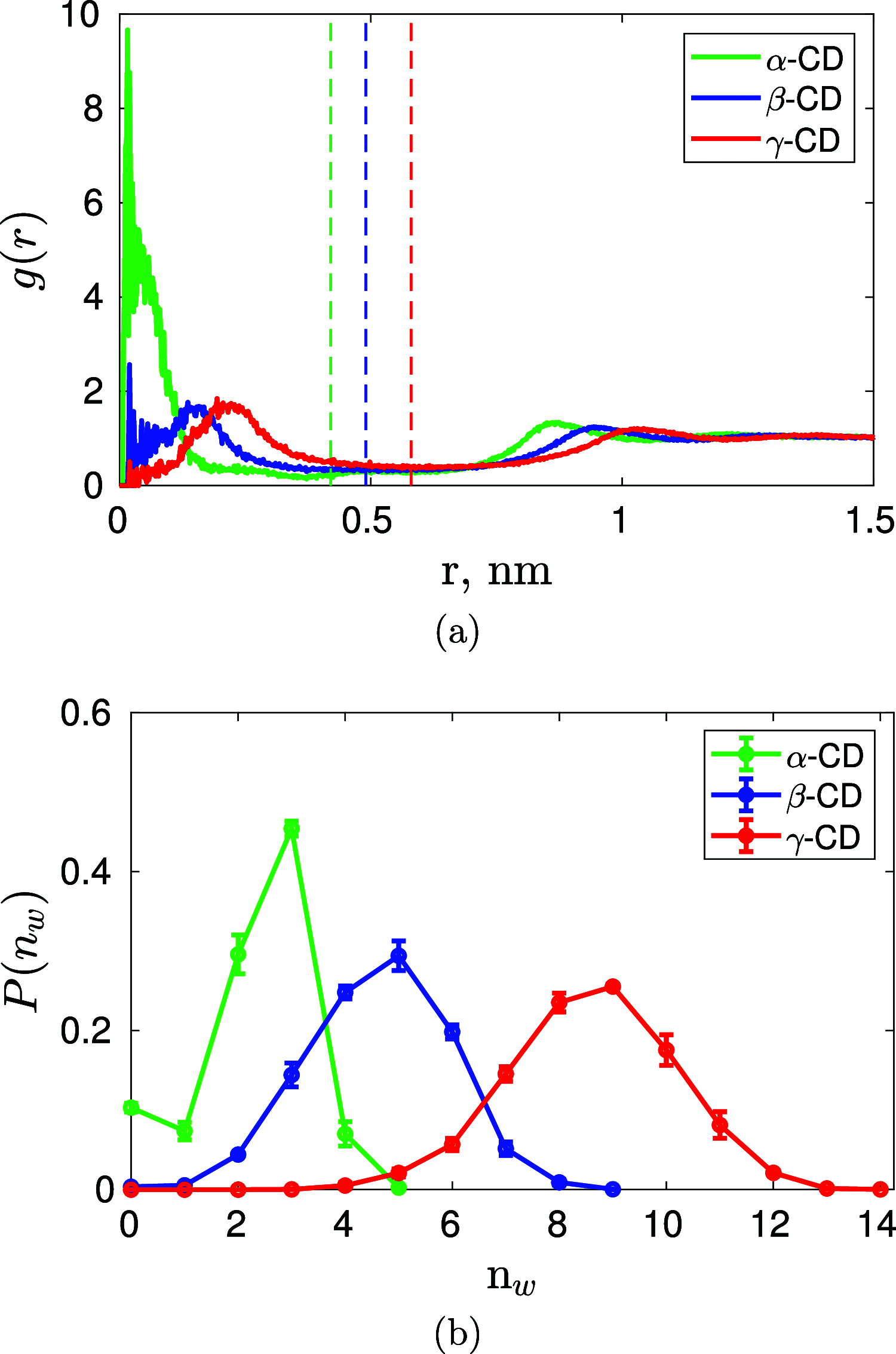
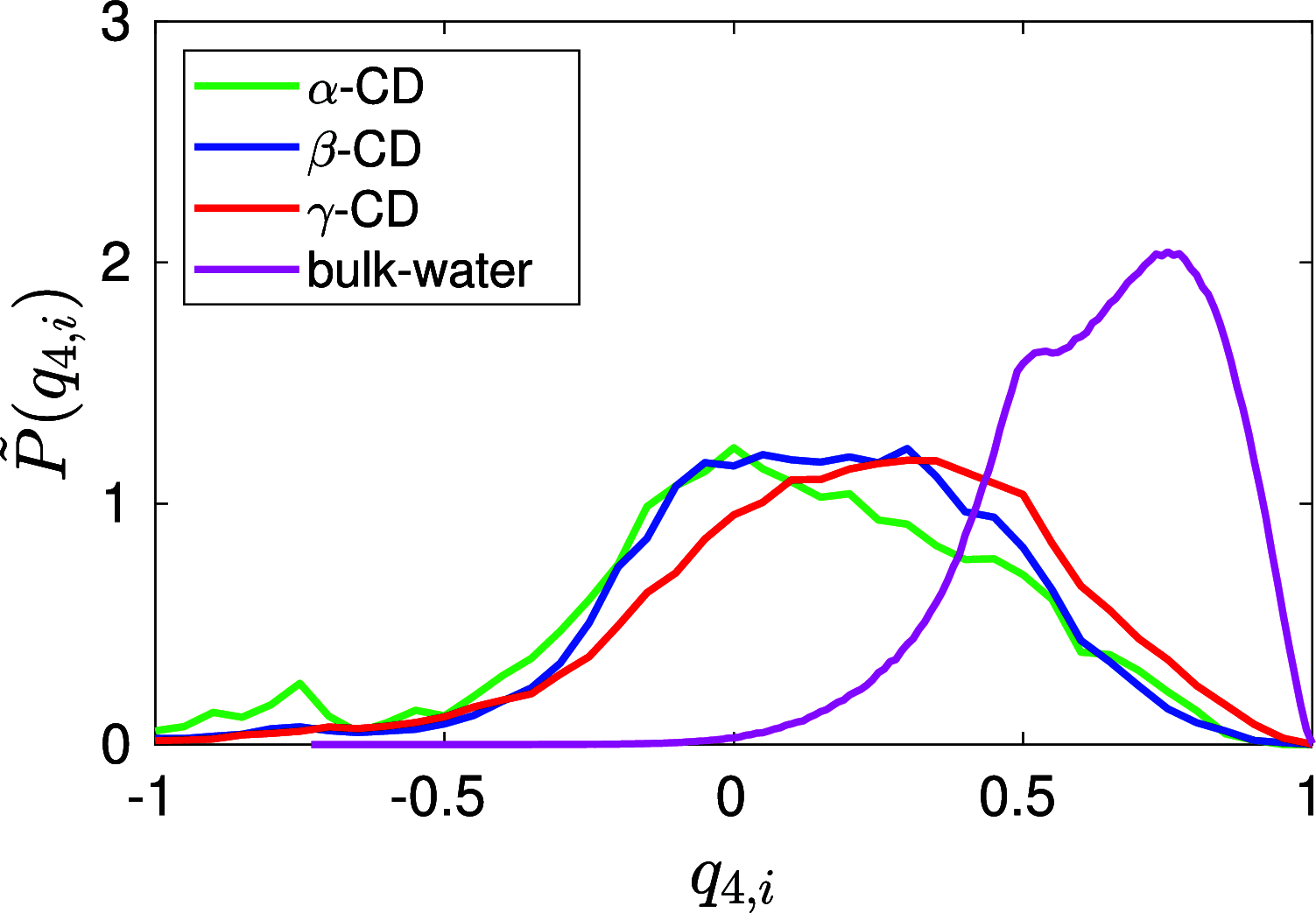
We find, through atomistic molecular dynamics simulation of native cyclodextrins (CDs) in water, that although the outer surface of a CD appears like a truncated cone, the inner cavity resembles a conical hourglass because of the inward protrusion of the glycosidic oxygens. Furthermore, the conformations of the constituent α-glucose molecules are found to differ significantly from a free monomeric α-glucose molecule. This is the first computational study that maps the conformational change to the preferential hydrogen bond donating capacity of one of the secondary hydroxyl groups of CD, in consensus with an NMR experiment. We have developed a simple and novel geometry-based technique to identify water molecules occupying the nonspherical CD cavity, and the computed water occupancies are in close agreement with the experimental and density functional theory studies. Our analysis reveals that a water molecule in CD cavity loses out about two hydrogen bonds and remains energetically frustrated but possesses higher orientational degree of freedom compared to bulk water. In the context of CD-drug complexation, these imply a nonclassical, that is, enthalpically driven hydrophobic association of a drug in CD cavity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
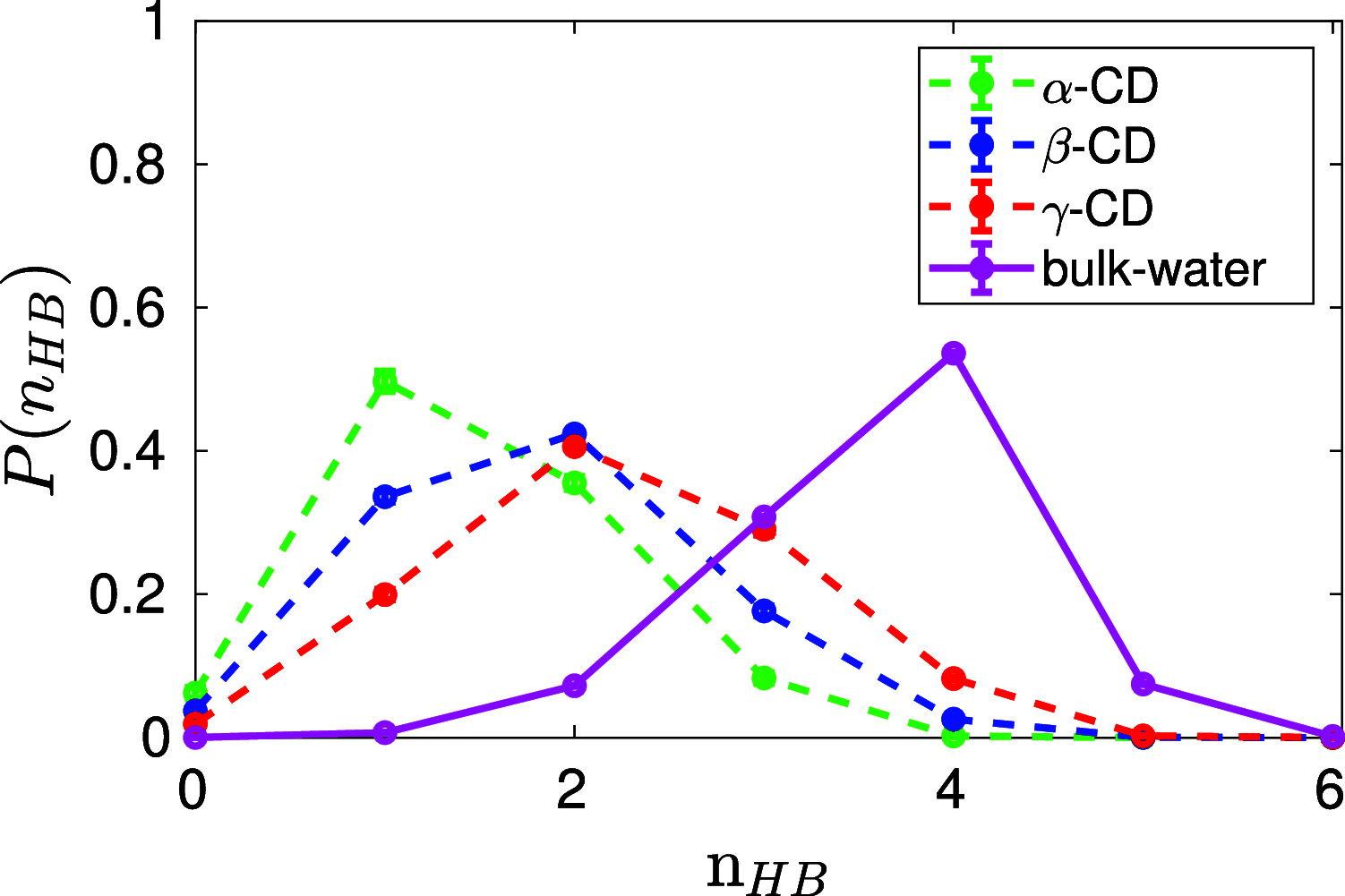
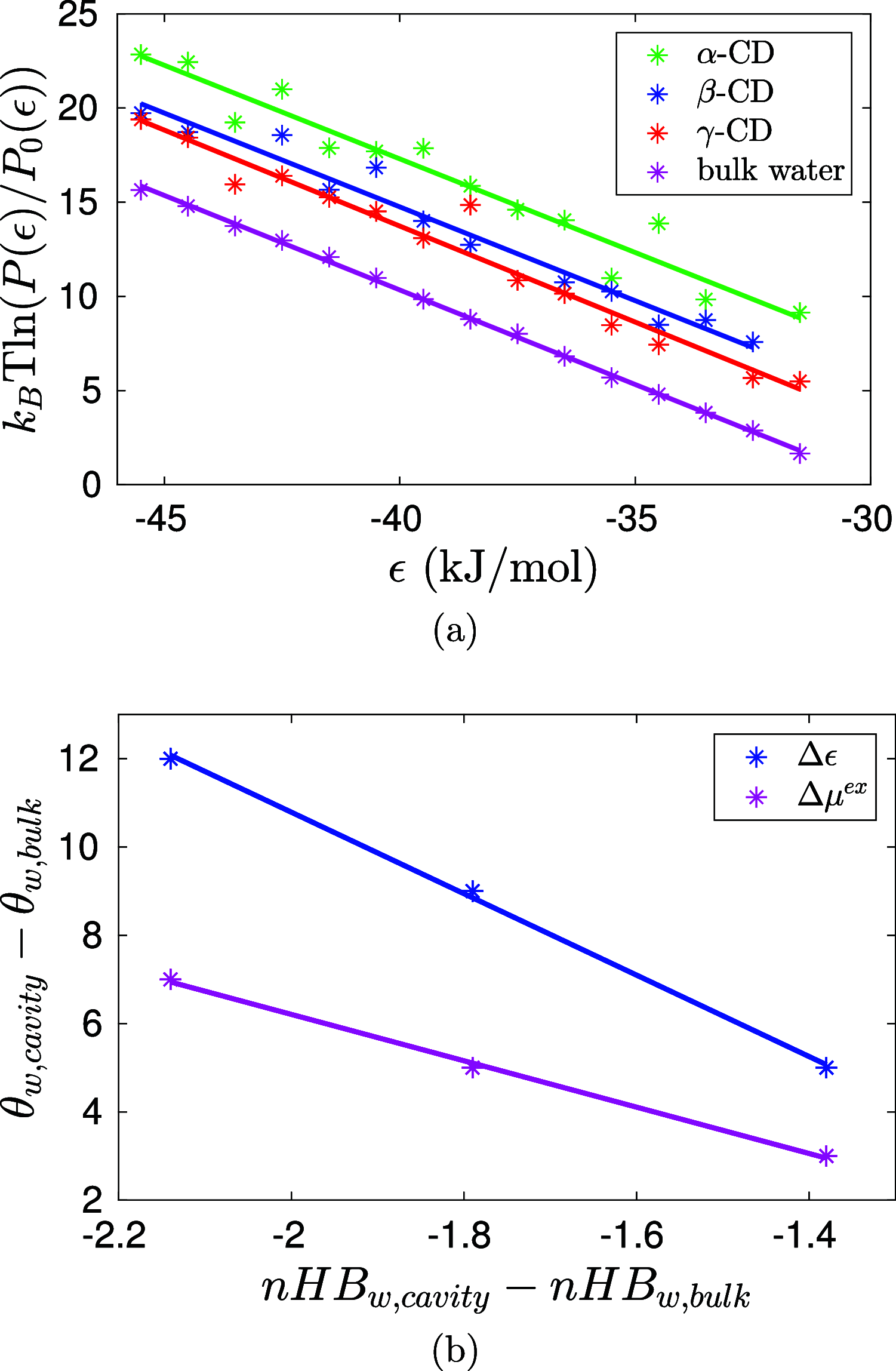
References
-
- Saenger W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem., Int. Ed. 1980, 19, 344–362. 10.1002/anie.198003441. - DOI
-
- Betzel C.; Saenger W.; Hingerty B. E.; Brown G. M. Topography of cyclodextrin inclusion complexes, part 20. Circular and flip-flop hydrogen bonding in .beta.-cyclodextrin undecahydrate: a neutron diffraction study. J. Am. Chem. Soc. 1984, 106, 7545–7557. 10.1021/ja00336a039. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

