The Balance between the Safety of Mother, Fetus, and Newborn Undergoing Cystic Fibrosis Transmembrane Conductance Regulator Treatments during Pregnancy
- PMID: 33073185
- PMCID: PMC7551705
- DOI: 10.1021/acsptsci.0c00098
The Balance between the Safety of Mother, Fetus, and Newborn Undergoing Cystic Fibrosis Transmembrane Conductance Regulator Treatments during Pregnancy
Abstract
The recent development of modulators of cystic fibrosis transmembrane conductance regulator (CFTR) has allowed the life expectancy of cystic fibrosis patients to increase substantially resulting in more women with cystic fibrosis reaching child-bearing age. This however raises the issue of whether long-term use of CFTR modulators during pregnancy and breastfeeding is safe for the fetus and newborn, especially for their developing brain. A very limited number of case reports available so far has shown that the fetus or breastfed newborn is likely to be exposed to maternally administered CFTR modulators. Potential impacts of drug exposure on the developing brain are of particular importance as the consequences might not be immediately noticeable upon birth but may manifest later in life as permanent neurobehavioral problems. In order for drugs in maternal circulation to enter the fetal brain, they must overcome the placental barrier followed by a series of brain barriers, each consisting of cellular components and physiological mechanisms such as efflux transporters. The extent of protection they offer during development will provide valuable insights into the potential entry and the effects of CFTR modulators in the developing brain. This review aims to explore the current understanding of the safety of CFTR modulators, especially ivacaftor, during pregnancy and breastfeeding, characterize the pharmacokinetics and pharmacodynamics of ivacaftor, both under normal conditions and during pregnancy, to provide context for its potential impact on the developing brain. Finally, we discuss the determinants that need to be taken into consideration when investigating the entry of drugs into the fetus and newborn.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
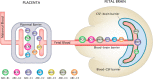
Similar articles
-
Emerging Cystic Fibrosis Transmembrane Conductance Regulator Modulators as New Drugs for Cystic Fibrosis: A Portrait of in Vitro Pharmacology and Clinical Translation.ACS Pharmacol Transl Sci. 2019 Oct 2;3(1):4-10. doi: 10.1021/acsptsci.9b00060. eCollection 2020 Feb 14. ACS Pharmacol Transl Sci. 2019. PMID: 32259083 Free PMC article. Review.
-
The Extrapulmonary Effects of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis.Ann Am Thorac Soc. 2020 Feb;17(2):147-154. doi: 10.1513/AnnalsATS.201909-671CME. Ann Am Thorac Soc. 2020. PMID: 31661636 Free PMC article. Review.
-
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function.Sci Rep. 2018 Nov 20;8(1):17066. doi: 10.1038/s41598-018-35151-7. Sci Rep. 2018. PMID: 30459435 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator modulators: Present and future in cystic fibrosis treatment. A review.Arch Argent Pediatr. 2019 Apr 1;117(2):e131-e136. doi: 10.5546/aap.2019.eng.e131. Arch Argent Pediatr. 2019. PMID: 30869491 Review. English, Spanish.
-
The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke.Am J Respir Cell Mol Biol. 2017 Jan;56(1):99-108. doi: 10.1165/rcmb.2016-0226OC. Am J Respir Cell Mol Biol. 2017. PMID: 27585394 Free PMC article.
Cited by
-
Multiple Reaction Monitoring Mass Spectrometry for the Drug Monitoring of Ivacaftor, Tezacaftor, and Elexacaftor Treatment Response in Cystic Fibrosis: A High-Throughput Method.ACS Pharmacol Transl Sci. 2020 Sep 11;3(5):987-996. doi: 10.1021/acsptsci.0c00103. eCollection 2020 Oct 9. ACS Pharmacol Transl Sci. 2020. PMID: 33073196 Free PMC article.
-
In Utero Mapping and Development Role of CFTR in Lung and Gastrointestinal Tract of Cystic Fibrosis Patients.ACS Pharmacol Transl Sci. 2023 Feb 13;6(3):355-360. doi: 10.1021/acsptsci.2c00233. eCollection 2023 Mar 10. ACS Pharmacol Transl Sci. 2023. PMID: 36926454 Free PMC article. Review.
-
Ocular development after highly effective modulator treatment early in life.Front Pharmacol. 2023 Sep 19;14:1265138. doi: 10.3389/fphar.2023.1265138. eCollection 2023. Front Pharmacol. 2023. PMID: 37795027 Free PMC article. Review.
-
Cystic Fibrosis Transmembrane Conductance Regulator Modulators During Pregnancy: A Case Series.Cureus. 2021 Aug 25;13(8):e17427. doi: 10.7759/cureus.17427. eCollection 2021 Aug. Cureus. 2021. PMID: 34589336 Free PMC article.
-
The choroid plexus: a missing link in our understanding of brain development and function.Physiol Rev. 2023 Jan 1;103(1):919-956. doi: 10.1152/physrev.00060.2021. Epub 2022 Sep 29. Physiol Rev. 2023. PMID: 36173801 Free PMC article. Review.
References
-
- Tan M.; Reyes-Ortega F.; Schneider-Futschik E. K. (2020) Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis. Magnetochemistry 6 (2), 25.10.3390/magnetochemistry6020025. - DOI
Publication types
LinkOut - more resources
Full Text Sources
