Abuse Liability, Anti-Nociceptive, and Discriminative Stimulus Properties of IBNtxA
- PMID: 33073190
- PMCID: PMC7551712
- DOI: 10.1021/acsptsci.0c00066
Abuse Liability, Anti-Nociceptive, and Discriminative Stimulus Properties of IBNtxA
Abstract
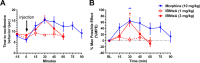
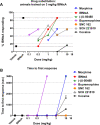
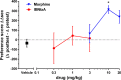
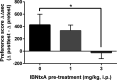
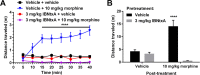
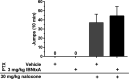
IBNtxA (3-iodobenzoyl naltrexamine) is a novel μ-opioid receptor (MOR) agonist which is structurally related to the MOR antagonist naltrexone. Recent studies suggest IBNtxA preferentially signals through truncated MOR splice variants, resulting in anti-nociception with reduced side effects, including no conditioned place preference (CPP) when tested at a single dose. IBNtxA represents an intriguing lead compound for preclinical drug development targeting truncated MOR splice variants, but further evaluation of its in vivo pharmacological profile is necessary. The purpose of this study was to independently verify the antinociceptive properties of IBNtxA and to examine more completely the rewarding properties and discriminative stimulus effects of IBNtxA, allowing broader assessment of IBNtxA as a candidate for further medications development. A dose of 3 mg/kg IBNtxA was equipotent to 10 mg/kg morphine in a hot-plate analgesia assay. In drug discrimination testing using mice trained to discriminate between 3 mg/kg IBNtxA and vehicle, the κ-agonist U-50488 fully substituted for IBNtxA. MOR agonist morphine, δ-agonist SNC162, NOP agonist SCH 221510, and MOR/NOP partial agonist buprenorphine each partially substituted for IBNtxA. IBNtxA up to 3 mg/kg did not produce a place preference in CPP. Pretreatment with 3 mg/kg IBNtxA but not 1 mg/kg IBNtxA attenuated acquisition of place preference for 10 mg/kg morphine. A dose of 3 mg/kg IBNtxA attenuated morphine-induced hyperlocomotion but did not alter naloxone-precipitated morphine withdrawal. Overall, IBNtxA has a complicated opioid receptor pharmacology in vivo. These results indicate that IBNtxA produces potent anti-nociception and has low abuse liability, likely driven by substantial κ agonist signaling effects.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
To probe interaction of morphine and IBNtxA with 7TM and 6TM variants of the human μ-opioid receptor using all-atom molecular dynamics simulations with an explicit membrane.Phys Chem Chem Phys. 2018 Jan 17;20(3):1724-1741. doi: 10.1039/c7cp06745c. Phys Chem Chem Phys. 2018. PMID: 29265141
-
Truncated μ-Opioid Receptors With 6 Transmembrane Domains Are Essential for Opioid Analgesia.Anesth Analg. 2018 Mar;126(3):1050-1057. doi: 10.1213/ANE.0000000000002538. Anesth Analg. 2018. PMID: 28991118 Free PMC article.
-
Synthesis and characterization of a dual kappa-delta opioid receptor agonist analgesic blocking cocaine reward behavior.ACS Chem Neurosci. 2015 Nov 18;6(11):1813-24. doi: 10.1021/acschemneuro.5b00153. Epub 2015 Sep 14. ACS Chem Neurosci. 2015. PMID: 26325040 Free PMC article.
-
Ligand-Free Signaling of G-Protein-Coupled Receptors: Relevance to μ Opioid Receptors in Analgesia and Addiction.Molecules. 2022 Sep 8;27(18):5826. doi: 10.3390/molecules27185826. Molecules. 2022. PMID: 36144565 Free PMC article. Review.
-
Current and Future Therapeutic Options in Pain Management: Multi-mechanistic Opioids Involving Both MOR and NOP Receptor Activation.CNS Drugs. 2022 Jun;36(6):617-632. doi: 10.1007/s40263-022-00924-2. Epub 2022 May 26. CNS Drugs. 2022. PMID: 35616826 Free PMC article. Review.
Cited by
-
Buprenorphine Pharmacodynamics: A Bridge to Understanding Buprenorphine Clinical Benefits.Drugs. 2025 Feb;85(2):215-230. doi: 10.1007/s40265-024-02128-y. Epub 2025 Jan 28. Drugs. 2025. PMID: 39873915 Review.
-
Advances in attenuating opioid-induced respiratory depression: A narrative review.Medicine (Baltimore). 2024 Jul 19;103(29):e38837. doi: 10.1097/MD.0000000000038837. Medicine (Baltimore). 2024. PMID: 39029082 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
