Efficacy of silk fibroin biomaterial vehicle for in vivo mucosal delivery of Griffithsin and protection against HIV and SHIV infection ex vivo
- PMID: 33073530
- PMCID: PMC7569169
- DOI: 10.1002/jia2.25628
Efficacy of silk fibroin biomaterial vehicle for in vivo mucosal delivery of Griffithsin and protection against HIV and SHIV infection ex vivo
Abstract
Introduction: The majority of new HIV infections occur through mucosal transmission. The availability of readily applicable and accessible platforms for anti-retroviral (ARV) delivery is critical for the prevention of HIV acquisition through sexual transmission in both women and men. There is a compelling need for developing new topical delivery systems that have advantages over the pills, gels and rings, which currently fail to guarantee protection against mucosal viral transmission in vulnerable populations due to lack of user compliance. The silk fibroin (SF) platform offers another option that may be better suited to individual circumstances and preferences to increase efficacy through user compliance. The objective of this study was to test safety and efficacy of SF for anti-HIV drug delivery to mucosal sites and for viral prevention.
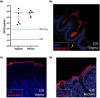
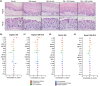
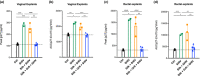
Methods: We formulated a potent HIV inhibitor Griffithsin (Grft) in a mucoadhesive silk fibroin (SF) drug delivery platform and tested the application in a non-human primate model in vivo and a pre-clinical human cervical and colorectal tissue explant model. Both vaginal and rectal compartments were assessed in rhesus macaques (Mucaca mulatta) that received SF (n = 4), no SF (n = 7) and SF-Grft (n = 11). In this study, we evaluated the composition of local microbiota, inflammatory cytokine production, histopathological changes in the vaginal and rectal compartments and mucosal protection after ex vivo SHIV challenge.
Results: Effective Grft release and retention in mucosal tissues from the SF-Grft platform resulted in protection against HIV in human cervical and colorectal tissue as well as against SHIV challenge in both rhesus macaque vaginal and rectal tissues. Mucoadhesion of SF-Grft inserts did not cause any inflammatory responses or changes in local microbiota.
Conclusions: We demonstrated that in vivo delivery of SF-Grft in rhesus macaques fully protects against SHIV challenge ex vivo after two hours of application and is safe to use in both the vaginal and rectal compartments. Our study provides support for the development of silk fibroin as a highly promising, user-friendly HIV prevention modality to address the global disparity in HIV infection.
Keywords: Mucaca mulatta; Griffithsin; HIV infections; biocompatible materials; silk fibroin; vulnerable populations.
© 2020 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
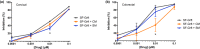
References
-
- Advocacy CaG . UNAIDS. 2019. [cited 2020 Jun 19]. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_...
-
- WHO . Focus on key populations in national HIV strategic plans in the African region. Congo: Regional Office for Africa; 2018.
-
- Akil A, Parniak MA, Dezzutti CS, Moncla BJ, Cost MR, Li M, et al. Development and characterization of a vaginal film containing dapivirine, a non‐nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV‐1 sexual transmission. Drug Delivery Transl Res. 2011;1(3):209–22. - PMC - PubMed

