Prolonged antithrombotic therapy in patients after acute coronary syndrome: A critical appraisal of current European Society of Cardiology guidelines
- PMID: 33073857
- PMCID: PMC8079117
- DOI: 10.5603/CJ.a2020.0132
Prolonged antithrombotic therapy in patients after acute coronary syndrome: A critical appraisal of current European Society of Cardiology guidelines
Abstract
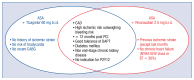
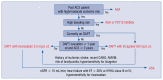
The increased risk of non-cardiovascular death in patients receiving clopidogrel or prasugrel in comparison with the placebo group in the Dual Antiplatelet Therapy (DAPT) trial in contrast to the decreased risk of cardiovascular death and all-cause death seen in patients treated with low-dose ticagrelor in the EU label population of the PEGASUS-TIMI 54 trial, resulted in inclusion in the 2020 ESC NSTE-ACS guidelines the recommendation for use of clopidogrel or prasugrel only if the patient is not eligible for treatment with ticagrelor. The prevalence of the primary outcome composed of cardiovascular death, stroke, or myocardial infarction was lower in the low-dose rivaroxaban and acetylsalicylic acid (ASA) group than in the ASA-alone group in the COMPASS trial. Moreover, all-cause mortality and cardiovascular mortality rates were lower in the rivaroxaban-plus-ASA group. Comparison of the PEGASUS-TIMI 54 and COMPASS trial patient characteristics clearly shows that each of these treatment strategies should be addressed at different groups of patients. A greater benefit in post-acute coronary syndrome (ACS) patients with a high risk of ischemic events and without high bleeding risk may be expected with ASA and ticagrelor 60 mg b.i.d. when the therapy is continued without interruption or with short interruption only after ACS. On the other hand, ASA and rivaroxaban 2.5 mg b.i.d. seems to be a better option when indications for dual antithrombotic therapy (DATT) appear after a longer time from ACS (more than 2 years) and/or from cessation of DAPT (more than 1 year) and in patients with multiple vascular bed atherosclerosis. Thus, both options of DATTs complement each other rather than compete, as can be presumed from the recommendations. However, a direct comparison between these strategies should be tested in future clinical trials.
Keywords: acute coronary syndrome; chronic coronary syndrome; clopidogrel; prasugrel; prolonged antithrombotic therapy; rivaroxaban; ticagrelor.
Conflict of interest statement
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

