Siderophore piracy enhances Vibrio cholerae environmental survival and pathogenesis
- PMID: 33074088
- PMCID: PMC7723260
- DOI: 10.1099/mic.0.000975
Siderophore piracy enhances Vibrio cholerae environmental survival and pathogenesis
Abstract
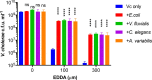
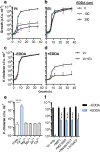
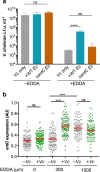
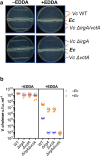
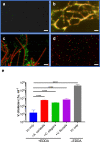
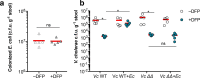
Vibrio cholerae, the aetiological agent of cholera, possesses multiple iron acquisition systems, including those for the transport of siderophores. How these systems benefit V. cholerae in low-iron, polymicrobial communities in environmental settings or during infection remains poorly understood. Here, we demonstrate that in iron-limiting conditions, co-culture of V. cholerae with a number of individual siderophore-producing microbes significantly promoted V. cholerae growth in vitro. We further show that in the host environment with low iron, V. cholerae colonizes better in adult mice in the presence of the siderophore-producing commensal Escherichia coli. Taken together, our results suggest that in aquatic reservoirs or during infection, V. cholerae may overcome environmental and host iron restriction by hijacking siderophores from other microbes.
Keywords: Anabaena variabilis; Cunninghamella elegans; Escherichia coli; Vibrio cholerae; Vibrio fluvialis; colonization; enterobactin; iron.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

