APOE2 is associated with longevity independent of Alzheimer's disease
- PMID: 33074098
- PMCID: PMC7588231
- DOI: 10.7554/eLife.62199
APOE2 is associated with longevity independent of Alzheimer's disease
Abstract
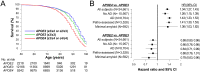
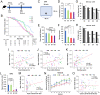
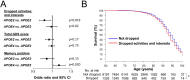
Although the ε2 allele of apolipoprotein E (APOE2) benefits longevity, its mechanism is not understood. The protective effects of the APOE2 on Alzheimer's disease (AD) risk, particularly through their effects on amyloid or tau accumulation, may confound APOE2 effects on longevity. Herein, we showed that the association between APOE2 and longer lifespan persisted irrespective of AD status, including its neuropathology, by analyzing clinical datasets as well as animal models. Notably, APOE2 was associated with preserved activity during aging, which also associated with lifespan. In animal models, distinct apoE isoform levels, where APOE2 has the highest, were correlated with activity levels, while some forms of cholesterol and triglycerides were associated with apoE and activity levels. These results indicate that APOE2 can contribute to longevity independent of AD. Preserved activity would be an early-observable feature of APOE2-mediated longevity, where higher levels of apoE2 and its-associated lipid metabolism might be involved.
Keywords: APOE; Alzheimer's disease; human; longevity; medicine; mouse; neuroscience.
© 2020, Shinohara et al.
Conflict of interest statement
MS, TK, MT, AK, MS, YF, JZ, XH, PS, GR, JF, MH, GB No competing interests declared
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

