Benfotiamine and Cognitive Decline in Alzheimer's Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial
- PMID: 33074237
- PMCID: PMC7880246
- DOI: 10.3233/JAD-200896
Benfotiamine and Cognitive Decline in Alzheimer's Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial
Abstract
Background: In preclinical models, benfotiamine efficiently ameliorates the clinical and biological pathologies that define Alzheimer's disease (AD) including impaired cognition, amyloid-β plaques, neurofibrillary tangles, diminished glucose metabolism, oxidative stress, increased advanced glycation end products (AGE), and inflammation.
Objective: To collect preliminary data on feasibility, safety, and efficacy in individuals with amnestic mild cognitive impairment (aMCI) or mild dementia due to AD in a placebo-controlled trial of benfotiamine.
Methods: A twelve-month treatment with benfotiamine tested whether clinical decline would be delayed in the benfotiamine group compared to the placebo group. The primary clinical outcome was the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). Secondary outcomes were the clinical dementia rating (CDR) score and fluorodeoxyglucose (FDG) uptake, measured with brain positron emission tomography (PET). Blood AGE were examined as an exploratory outcome.
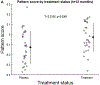
Results: Participants were treated with benfotiamine (34) or placebo (36). Benfotiamine treatment was safe. The increase in ADAS-Cog was 43% lower in the benfotiamine group than in the placebo group, indicating less cognitive decline, and this effect was nearly statistically significant (p = 0.125). Worsening in CDR was 77% lower (p = 0.034) in the benfotiamine group compared to the placebo group, and this effect was stronger in the APOEɛ4 non-carriers. Benfotiamine significantly reduced increases in AGE (p = 0.044), and this effect was stronger in the APOEɛ4 non-carriers. Exploratory analysis derivation of an FDG PET pattern score showed a treatment effect at one year (p = 0.002).
Conclusion: Oral benfotiamine is safe and potentially efficacious in improving cognitive outcomes among persons with MCI and mild AD.
Keywords: Advanced glycation endproducts; Alzheimer’s disease; benfotiamine; glucose; inflammation; oxidative stress.
Figures



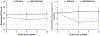







References
-
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR, Hornbeck RC, Paumier KL, Ances BM, Berman SB, Brickman AM, Cash DM, Chhatwal JP, Correia S, Förster S, Fox NC, Graff-Radford NR, la Fougère C, Levin J, Masters CL, Rossor MN, Salloway S, Saykin AJ, Schofield PR, Thompson PM, Weiner MM, Holtzman DM, Raichle ME, Morris JC, Bateman RJ, Benzinger TLS (2018) Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol 17, 241–250. - PMC - PubMed
-
- Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT (2020) Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med 26, 769–780. - PMC - PubMed
-
- Gibson GE, Sheu K-FR, Blass JP, Baker A, Carlson KC, Harding B, Perrino P (1988) Reduced Activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol 45, 836–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

