Short-term Outcomes of the Study of Refeeding to Optimize Inpatient Gains for Patients With Anorexia Nervosa: A Multicenter Randomized Clinical Trial
- PMID: 33074282
- PMCID: PMC7573797
- DOI: 10.1001/jamapediatrics.2020.3359
Short-term Outcomes of the Study of Refeeding to Optimize Inpatient Gains for Patients With Anorexia Nervosa: A Multicenter Randomized Clinical Trial
Abstract
Importance: The standard of care for refeeding inpatients with anorexia nervosa, starting with low calories and advancing cautiously, is associated with slow weight gain and protracted hospital stay. Limited data suggest that higher-calorie refeeding improves these outcomes with no increased risk of refeeding syndrome.
Objective: To compare the short-term efficacy, safety, and cost of lower-calorie vs higher-calorie refeeding for malnourished adolescents and young adults with anorexia nervosa.
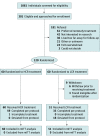
Design, setting, and participants: In this multicenter randomized clinical trial with prospective follow-up conducted at 2 inpatient eating disorder programs at large tertiary care hospitals, 120 adolescents and young adults aged 12 to 24 years hospitalized with anorexia nervosa or atypical anorexia nervosa and 60% or more of median body mass index were enrolled from February 8, 2016, to March 7, 2019. The primary analysis was a modified intent-to-treat approach.
Interventions: Higher-calorie refeeding, beginning at 2000 kcal/d and increasing by 200 kcal/d vs lower-calorie refeeding, beginning at 1400 k/cal and increasing by 200 kcal every other day.
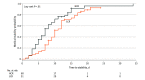
Main outcomes and measures: Main outcomes were end-of-treatment outcomes; the primary end point of this trial will be clinical remission over 12 months. Short-term efficacy was defined a priori as time to restore medical stability in the hospital, measured by the following 6 indices: 24-hour heart rate of 45 beats/min or more, systolic blood pressure of 90 mm Hg or more, temperature of 35.6 °C or more, orthostatic increase in heart rate of 35 beats/min or less, orthostatic decrease in systolic blood pressure of 20 mm Hg or less, and 75% or more of median body mass index for age and sex. The prespecified safety outcome was incidence of electrolyte abnormalities; cost efficacy was defined as savings associated with length of stay.
Results: Because 9 participants withdrew prior to treatment, the modified intention-to-treat analyses included 111 participants (93%; 101 females [91%]; mean [SD] age, 16.4 [2.5] years). Higher-calorie refeeding restored medical stability significantly earlier than lower-calorie refeeding (hazard ratio, 1.67 [95% CI, 1.10-2.53]; P = .01). Electrolyte abnormalities and other adverse events did not differ by group. Hospital stay was 4.0 days shorter (95% CI, -6.1 to -1.9 days) among the group receiving higher-calorie refeeding, which was associated with a savings of $19 056 (95% CI, -$28 819 to -$9293) in hospital charges per participant.
Conclusions and relevance: In the first randomized clinical trial in the US to compare refeeding approaches in patients with anorexia nervosa and atypical anorexia nervosa, higher-calorie refeeding demonstrated short-term efficacy with no increase in safety events during hospitalization.
Trial registration: ClinicalTrials.gov Identifier: NCT02488109.
Conflict of interest statement
Figures

Comment in
-
Higher-calorie vs lower-calorie refeeding in patients with anorexia improves time to medical stability without compromising safety.J Pediatr. 2021 May;232:307-310. doi: 10.1016/j.jpeds.2021.02.054. J Pediatr. 2021. PMID: 33896456 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

