Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-analysis
- PMID: 33074286
- PMCID: PMC7573798
- DOI: 10.1001/jamaneurol.2020.3536
Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-analysis
Erratum in
-
Error in Author Name.JAMA Neurol. 2021 May 1;78(5):620. doi: 10.1001/jamaneurol.2021.0456. JAMA Neurol. 2021. PMID: 33749757 Free PMC article. No abstract available.
Abstract
Importance: Compared with non-Hispanic White individuals, African American individuals from the same community are approximately twice as likely to develop Alzheimer disease. Despite this disparity, the largest Alzheimer disease genome-wide association studies to date have been conducted in non-Hispanic White individuals. In the largest association analyses of Alzheimer disease in African American individuals, ABCA7, TREM2, and an intergenic locus at 5q35 were previously implicated.
Objective: To identify additional risk loci in African American individuals by increasing the sample size and using the African Genome Resource panel.
Design, setting, and participants: This genome-wide association meta-analysis used case-control and family-based data sets from the Alzheimer Disease Genetics Consortium. There were multiple recruitment sites throughout the United States that included individuals with Alzheimer disease and controls of African American ancestry. Analysis began October 2018 and ended September 2019.
Main outcomes and measures: Diagnosis of Alzheimer disease.
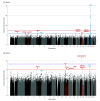
Results: A total of 2784 individuals with Alzheimer disease (1944 female [69.8%]) and 5222 controls (3743 female [71.7%]) were analyzed (mean [SD] age at last evaluation, 74.2 [13.6] years). Associations with 4 novel common loci centered near the intracellular glycoprotein trafficking gene EDEM1 (3p26; P = 8.9 × 10-7), near the immune response gene ALCAM (3q13; P = 9.3 × 10-7), within GPC6 (13q31; P = 4.1 × 10-7), a gene critical for recruitment of glutamatergic receptors to the neuronal membrane, and within VRK3 (19q13.33; P = 3.5 × 10-7), a gene involved in glutamate neurotoxicity, were identified. In addition, several loci associated with rare variants, including a genome-wide significant intergenic locus near IGF1R at 15q26 (P = 1.7 × 10-9) and 6 additional loci with suggestive significance (P ≤ 5 × 10-7) such as API5 at 11p12 (P = 8.8 × 10-8) and RBFOX1 at 16p13 (P = 5.4 × 10-7) were identified. Gene expression data from brain tissue demonstrate association of ALCAM, ARAP1, GPC6, and RBFOX1 with brain β-amyloid load. Of 25 known loci associated with Alzheimer disease in non-Hispanic White individuals, only APOE, ABCA7, TREM2, BIN1, CD2AP, FERMT2, and WWOX were implicated at a nominal significance level or stronger in African American individuals. Pathway analyses strongly support the notion that immunity, lipid processing, and intracellular trafficking pathways underlying Alzheimer disease in African American individuals overlap with those observed in non-Hispanic White individuals. A new pathway emerging from these analyses is the kidney system, suggesting a novel mechanism for Alzheimer disease that needs further exploration.
Conclusions and relevance: While the major pathways involved in Alzheimer disease etiology in African American individuals are similar to those in non-Hispanic White individuals, the disease-associated loci within these pathways differ.
Conflict of interest statement
Figures
References
-
- Hollingworth P, Harold D, Sims R, et al. ; Alzheimer’s Disease Neuroimaging Initiative; CHARGE consortium; EADI1 consortium . Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429-435. doi: 10.1038/ng.803 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG062428/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- P30 AG066518/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG059307/AG/NIA NIH HHS/United States
- R01 AG009029/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 AG009956/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- RC2 AG036650/AG/NIA NIH HHS/United States
- R01 AG019085/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- P30 AG066515/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG030146/AG/NIA NIH HHS/United States
- RF1 AG066107/AG/NIA NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- U24 AG056270/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- R01 AG028786/AG/NIA NIH HHS/United States
- KL2 RR024151/RR/NCRR NIH HHS/United States
- K25 AG055620/AG/NIA NIH HHS/United States
- RF1 AG054023/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- RF1 AG054080/AG/NIA NIH HHS/United States
- RF1 AG051504/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- R01 AG061796/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R37 AG015473/AG/NIA NIH HHS/United States
- P01 AG060882/AG/NIA NIH HHS/United States
- U24 AG026395/AG/NIA NIH HHS/United States
- U01 AG052410/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- R01 AG037212/AG/NIA NIH HHS/United States
- U01 AG046139/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- R01 AG027944/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- U01 HG004610/HG/NHGRI NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- U01 AG058635/AG/NIA NIH HHS/United States
- P30 AG072959/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

