Comparison of First-Line Treatments for Patients With Extensive-Stage Small Cell Lung Cancer: A Systematic Review and Network Meta-analysis
- PMID: 33074323
- PMCID: PMC7573680
- DOI: 10.1001/jamanetworkopen.2020.15748
Comparison of First-Line Treatments for Patients With Extensive-Stage Small Cell Lung Cancer: A Systematic Review and Network Meta-analysis
Abstract
Importance: Combinations of chemotherapy with immunotherapy or bevacizumab in first-line treatments of extensive-stage small cell lung cancer (ES-SCLC) have been evaluated in various clinical trials. However, it remains unclear what the optimal combination regimen is.
Objective: To clarify which first-line combination regimen is associated with the best tumor response among patients with ES-SCLC.
Data sources: Electronic databases (PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science) were systematically searched to extract eligible literature from database inception to December 2019.
Study selection: Head-to-head randomized clinical trials on first-line treatments for patients with ES-SCLC were included with outcomes and toxic effects reported, including objective response rate (ORR, involving complete response and partial response), disease control rate (DCR, involving complete response, partial response, and stable disease), progression-free survival (PFS), overall survival (OS), and treatment related adverse events (TRAEs) of grades 3 to 5. Of 199 eligible articles, 14 were included.
Data extraction and synthesis: Data were independently extracted and collected by 2 reviewers based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Data were pooled using a random-effects model.
Main outcomes and measures: Main outcomes were OS, PFS, DCR, ORR, and TRAEs of grades 3 to 5.
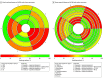
Results: A total of 3 phase 2 and 11 phase 3 randomized clinical trials involving 4838 patients were included. Programmed cell death ligand 1 (PD-L1) inhibitor (durvalumab and atezolizumab) plus etoposide-based chemotherapy, compared with etoposide-based chemotherapy alone, showed the most favorable OS (hazard ratio, 1.40; 95% CI, 1.09-1.80) and the best DCR (odds ratio [OR], 0.42; 95% CI, 0.21-0.81). Bevacizumab plus etoposide-based chemotherapy provided the best PFS compared with etoposide-based chemotherapy alone (hazard ratio, 1.54; 95% CI, 1.09-2.27), although this was not translated into OS benefit. The addition of PD-L1 inhibitors to etoposide-platinum chemotherapy caused no more toxic effects in general (compared with etoposide-based chemotherapy alone: OR, 1.14; 95% CI, 0.36-2.31), while bevacizumab plus etoposide-platinum regimen induced the most TRAEs grades 3 to 5 among all first-line treatments (eg, compared with irinotecan-platinum regimen: OR, 4.24; 95% CI, 1.26-14.57). Based on the surface under the cumulative ranking curve value, PD-L1 inhibitor plus etoposide-platinum had the highest probability of being ranked first for OS (0.87) and DCR (0.97).
Conclusions and relevance: The findings of this systematic review and network meta-analysis suggest that the combination of a PD-L1 inhibitor (durvalumab and atezolizumab) and etoposide-based chemotherapy may be an optimal first-line treatment option for patients with ES-SCLC patients.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

