Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial
- PMID: 33074327
- PMCID: PMC7573681
- DOI: 10.1001/jamanetworkopen.2020.20836
Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial
Abstract
Importance: The potential benefit of novel skeletal muscle anabolic agents to improve physical function in people with sarcopenia and other muscle wasting diseases is unknown.
Objective: To confirm the safety and efficacy of bimagrumab plus the new standard of care on skeletal muscle mass, strength, and physical function compared with standard of care alone in community-dwelling older adults with sarcopenia.
Design, setting, and participants: This double-blind, placebo-controlled, randomized clinical trial was conducted at 38 sites in 13 countries among community-dwelling men and women aged 70 years and older meeting gait speed and skeletal muscle criteria for sarcopenia. The study was conducted from December 2014 to June 2018, and analyses were conducted from August to November 2018.
Interventions: Bimagrumab 700 mg or placebo monthly for 6 months with adequate diet and home-based exercise.
Main outcomes and measures: The primary outcome was the change in Short Physical Performance Battery (SPPB) score after 24 weeks of treatment. Secondary outcomes included 6-minute walk distance, usual gait speed, handgrip strength, lean body mass, fat body mass, and standard safety parameters.
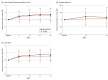
Results: A total of 180 participants were recruited, with 113 randomized to bimagrumab and 67 randomized to placebo. Among these, 159 participants (88.3%; mean [SD] age, 79.1 [5.3] years; 109 [60.6%] women) completed the study. The mean SPPB score increased by a mean of 1.34 (95% CI, 0.90 to 1.77) with bimagrumab vs 1.03 (95% CI, 0.53 to 1.52) with placebo (P = .13); 6-minute walk distance increased by a mean of 24.60 (95% CI, 7.65 to 41.56) m with bimagrumab vs 14.30 (95% CI, -4.64 to 33.23) m with placebo (P = .16); and gait speed increased by a mean of 0.14 (95% CI, 0.09 to 0.18) m/s with bimagrumab vs 0.11 (95% CI, 0.05 to 0.16) m/s with placebo (P = .16). Bimagrumab was safe and well-tolerated and increased lean body mass by 7% (95% CI, 6% to 8%) vs 1% (95% CI, 0% to 2%) with placebo, resulting in difference of 6% (95% CI, 4% to 7%) (P < .001).
Conclusions and relevance: This randomized clinical trial found no significant difference between participants treated with bimagrumab vs placebo among older adults with sarcopenia who had 6 months of adequate nutrition and light exercise, with physical function improving in both groups. Bimagrumab treatment was safe, well-tolerated, increased lean body mass, and decreased fat body mass. The effects of sarcopenia, an increasing cause of disability in older adults, can be reduced with proper diet and exercise.
Trial registration: ClinicalTrials.gov Identifier: NCT02333331; EudraCT number: 2014-003482-25.
Conflict of interest statement
Figures

References
-
- He W, Goodkind D, Kowal P.. An Aging World: 2015. US Census Bureau, US Government Publishing Office; 2016.
-
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. doi:10.1093/ageing/afy169 - DOI - PMC - PubMed
-
- Vellas B, Fielding RA, Bens C, et al. . Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2018;7(1):2-9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

