Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation
- PMID: 33074715
- PMCID: PMC7780997
- DOI: 10.1165/rcmb.2020-0360OC
Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation
Abstract
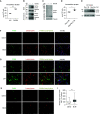
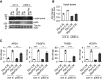
Augmented glycolysis due to metabolic reprogramming in lung myofibroblasts is critical to their profibrotic phenotype. The primary glycolysis byproduct, lactate, is also secreted into the extracellular milieu, together with which myofibroblasts and macrophages form a spatially restricted site usually described as fibrotic niche. Therefore, we hypothesized that myofibroblast glycolysis might have a non-cell autonomous effect through lactate regulating the pathogenic phenotype of alveolar macrophages. Here, we demonstrated that there was a markedly increased lactate in the conditioned media of TGF-β1 (transforming growth factor-β1)-induced lung myofibroblasts and in the BAL fluids (BALFs) from mice with TGF-β1- or bleomycin-induced lung fibrosis. Importantly, the media and BALFs promoted profibrotic mediator expression in macrophages. Mechanistically, lactate induced histone lactylation in the promoters of the profibrotic genes in macrophages, consistent with the upregulation of this epigenetic modification in these cells in the fibrotic lungs. The lactate inductions of the histone lactylation and profibrotic gene expression were mediated by p300, as evidenced by their diminished concentrations in p300-knockdown macrophages. Collectively, our study establishes that in addition to protein, lipid, and nucleic acid molecules, a metabolite can also mediate intercellular regulations in the setting of lung fibrosis. Our findings shed new light on the mechanism underlying the key contribution of myofibroblast glycolysis to the pathogenesis of lung fibrosis.
Keywords: histone lactylation; lactate; macrophage; myofibroblast; pulmonary fibrosis.
Figures





Comment in
-
Myofibroblast-Macrophage Interactions Turn Sour in Fibrotic Lungs.Am J Respir Cell Mol Biol. 2021 Jan;64(1):14-15. doi: 10.1165/rcmb.2020-0473ED. Am J Respir Cell Mol Biol. 2021. PMID: 33166479 Free PMC article. No abstract available.
References
-
- Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–688. - PubMed
-
- Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov. 2017;16:755–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

