The impact of delayed treatment of uncomplicated P. falciparum malaria on progression to severe malaria: A systematic review and a pooled multicentre individual-patient meta-analysis
- PMID: 33075101
- PMCID: PMC7571702
- DOI: 10.1371/journal.pmed.1003359
The impact of delayed treatment of uncomplicated P. falciparum malaria on progression to severe malaria: A systematic review and a pooled multicentre individual-patient meta-analysis
Abstract
Background: Delay in receiving treatment for uncomplicated malaria (UM) is often reported to increase the risk of developing severe malaria (SM), but access to treatment remains low in most high-burden areas. Understanding the contribution of treatment delay on progression to severe disease is critical to determine how quickly patients need to receive treatment and to quantify the impact of widely implemented treatment interventions, such as 'test-and-treat' policies administered by community health workers (CHWs). We conducted a pooled individual-participant meta-analysis to estimate the association between treatment delay and presenting with SM.
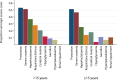
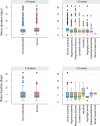
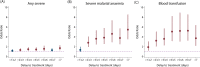
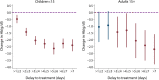
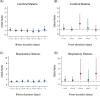
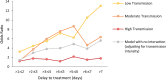
Methods and findings: A search using Ovid MEDLINE and Embase was initially conducted to identify studies on severe Plasmodium falciparum malaria that included information on treatment delay, such as fever duration (inception to 22nd September 2017). Studies identified included 5 case-control and 8 other observational clinical studies of SM and UM cases. Risk of bias was assessed using the Newcastle-Ottawa scale, and all studies were ranked as 'Good', scoring ≥7/10. Individual-patient data (IPD) were pooled from 13 studies of 3,989 (94.1% aged <15 years) SM patients and 5,780 (79.6% aged <15 years) UM cases in Benin, Malaysia, Mozambique, Tanzania, The Gambia, Uganda, Yemen, and Zambia. Definitions of SM were standardised across studies to compare treatment delay in patients with UM and different SM phenotypes using age-adjusted mixed-effects regression. The odds of any SM phenotype were significantly higher in children with longer delays between initial symptoms and arrival at the health facility (odds ratio [OR] = 1.33, 95% CI: 1.07-1.64 for a delay of >24 hours versus ≤24 hours; p = 0.009). Reported illness duration was a strong predictor of presenting with severe malarial anaemia (SMA) in children, with an OR of 2.79 (95% CI:1.92-4.06; p < 0.001) for a delay of 2-3 days and 5.46 (95% CI: 3.49-8.53; p < 0.001) for a delay of >7 days, compared with receiving treatment within 24 hours from symptom onset. We estimate that 42.8% of childhood SMA cases and 48.5% of adult SMA cases in the study areas would have been averted if all individuals were able to access treatment within the first day of symptom onset, if the association is fully causal. In studies specifically recording onset of nonsevere symptoms, long treatment delay was moderately associated with other SM phenotypes (OR [95% CI] >3 to ≤4 days versus ≤24 hours: cerebral malaria [CM] = 2.42 [1.24-4.72], p = 0.01; respiratory distress syndrome [RDS] = 4.09 [1.70-9.82], p = 0.002). In addition to unmeasured confounding, which is commonly present in observational studies, a key limitation is that many severe cases and deaths occur outside healthcare facilities in endemic countries, where the effect of delayed or no treatment is difficult to quantify.
Conclusions: Our results quantify the relationship between rapid access to treatment and reduced risk of severe disease, which was particularly strong for SMA. There was some evidence to suggest that progression to other severe phenotypes may also be prevented by prompt treatment, though the association was not as strong, which may be explained by potential selection bias, sample size issues, or a difference in underlying pathology. These findings may help assess the impact of interventions that improve access to treatment.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: PH works for Medicines for Malaria Venture (MMV), which has a Research Collaboration Agreement in place with Imperial College. LCO declares grant funding from the World Health Organization, the Bill and Melinda Gates Foundation, and Medicines for Malaria Venture.
Figures
References
-
- World Health Organization. World Malaria Report 2019. 2019. [cited 2020 Jan 17]. Available from: https://www.who.int/publications/i/item/world-malaria-report-2019
-
- Bennett A, Bisanzio D, Yukich JO, Mappin B, Fergus CA, Lynch M, et al. Population coverage of artemisinin-based combination treatment in children younger than 5 years with fever and Plasmodium falciparum infection in Africa, 2003–2015: a modelling study using data from national surveys. The Lancet Global health. 2017;5(4): e418–e27. 10.1016/S2214-109X(17)30076-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- D43 NS078280/NS/NINDS NIH HHS/United States
- G98669/MRC_/Medical Research Council/United Kingdom
- MR/L006529/1/MRC_/Medical Research Council/United Kingdom
- R01 NS055349/NS/NINDS NIH HHS/United States
- G9901439/MRC_/Medical Research Council/United Kingdom
- UH1 HL003679/HL/NHLBI NIH HHS/United States
- R01 AI044857/AI/NIAID NIH HHS/United States
- G0701427/MRC_/Medical Research Council/United Kingdom
- R01 AI116472/AI/NIAID NIH HHS/United States
- R21 TW006794/TW/FIC NIH HHS/United States
- M01 RR010284/RR/NCRR NIH HHS/United States
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

