FMS-like tyrosine kinase 3 (FLT3) amplification in patients with metastatic colorectal cancer
- PMID: 33075166
- PMCID: PMC7780005
- DOI: 10.1111/cas.14693
FMS-like tyrosine kinase 3 (FLT3) amplification in patients with metastatic colorectal cancer
Abstract
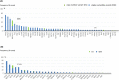
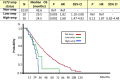
FMS-like tyrosine kinase 3 (FLT3) plays a key role in hematopoiesis. However, the oncogenic role of FLT3 amplification in patients with metastatic colorectal cancer (mCRC) remains unclear. Here, we aimed to evaluate the characteristics, prognosis, and treatment efficacy of an FLT3 inhibitor (regorafenib) in patients with mCRC with FLT3 amplifications. Tumor tissue samples from 2329 patients were sequenced using NGS in the Nationwide Cancer Genome Screening Project in Japan. The effects of clinicopathological features, co-altered genes, prognosis, and efficacy of regorafenib were investigated. Between April 2015 and June 2018, 85 patients with mCRC with FLT3 amplification were observed. There were no differences in baseline characteristics between patients with or without FLT3 amplification. The frequency of RAS or other gene co-alterations was inversely correlated with the copy number status. Median survival time in patients with FLT3 amplification was significantly shorter compared with those with non-FLT3 amplification. Further investigations of FLT3 amplification as a potential treatment target in mCRC are warranted.
Keywords: FLT3 amplification; colorectal cancer; copy number status; next-generation sequencing; prognosis.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Hiroya Taniguchi received research funding from Eli Lilly Japan Co., Ltd., Takeda, and Daiichi Sankyo. Yoshiaki Nakamura received research funding from Taiho Pharmaceutical. Satoshi Yuki received research funding from Chugai Pharmaceutical Co., Ltd. and Eli Lilly Japan Co., Ltd. Toshikazu Moriwaki received research honoraria from Taiho Pharmaceutical Co., Ltd and Chugai Pharmaceutical Co., Ltd. Eiji Oki received honoraria from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Eli Lilly Japan Co., Ltd. Akihito Tuji received honoraria from Taiho Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd. Takayuki Yoshino received research funding from Novartis Pharma KK, Chugai Pharmaceutical Co., Ltd., and Daiichi Sankyo. All other authors have nothing to declare.
Figures

Similar articles
-
Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial.Lancet Oncol. 2015 Aug;16(8):937-48. doi: 10.1016/S1470-2045(15)00138-2. Epub 2015 Jul 13. Lancet Oncol. 2015. PMID: 26184520 Free PMC article.
-
Eligibility of real-world patients with chemo-refractory, K-RAS wild-type, metastatic colorectal cancer for palliative intent regorafenib monotherapy.Med Oncol. 2018 Jun 23;35(8):114. doi: 10.1007/s12032-018-1176-6. Med Oncol. 2018. PMID: 29936654
-
The implication of FLT3 amplification for FLT targeted therapeutics in solid tumors.Oncotarget. 2017 Jan 10;8(2):3237-3245. doi: 10.18632/oncotarget.13700. Oncotarget. 2017. PMID: 27906677 Free PMC article.
-
Regorafenib: a novel multitargeted tyrosine kinase inhibitor for colorectal cancer and gastrointestinal stromal tumors.Ann Pharmacother. 2013 Dec;47(12):1685-96. doi: 10.1177/1060028013509792. Epub 2013 Nov 1. Ann Pharmacother. 2013. PMID: 24259629 Review.
-
Regorafenib: A Review in Metastatic Colorectal Cancer.Drugs. 2018 Jul;78(11):1133-1144. doi: 10.1007/s40265-018-0938-y. Drugs. 2018. PMID: 29943375 Review.
Cited by
-
CLG promotes mTOR/ULK1 pathway-mediated autophagy to inhibit OS development by inhibiting TRAF6-mediated FLT3 ubiquitination.Cancer Sci. 2024 Oct;115(10):3466-3480. doi: 10.1111/cas.16274. Epub 2024 Aug 9. Cancer Sci. 2024. PMID: 39118482 Free PMC article.
-
Case Report: Progressive disease of BRCA2-mutant colon adenocarcinoma following talazoparib therapy.Front Oncol. 2023 Nov 7;13:1245547. doi: 10.3389/fonc.2023.1245547. eCollection 2023. Front Oncol. 2023. PMID: 38023256 Free PMC article.
-
FLT3-Mutated Leukemic Stem Cells: Mechanisms of Resistance and New Therapeutic Targets.Cancers (Basel). 2024 May 10;16(10):1819. doi: 10.3390/cancers16101819. Cancers (Basel). 2024. PMID: 38791898 Free PMC article. Review.
-
Targeting PKC as a Therapeutic Strategy to Overcome Chemoresistance in TNBC by Restoring Aurora Kinase B Expression.J Cell Mol Med. 2025 Mar;29(6):e70464. doi: 10.1111/jcmm.70464. J Cell Mol Med. 2025. PMID: 40099930 Free PMC article.
-
Case report: Precision guided reactive cancer management: molecular complete response in heavily pretreated metastatic CRC by dual immunotherapy and sorafenib.Front Oncol. 2024 Jul 1;14:1405170. doi: 10.3389/fonc.2024.1405170. eCollection 2024. Front Oncol. 2024. PMID: 39011472 Free PMC article.
References
-
- Blume‐Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:55‐365. - PubMed
-
- Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147‐161. - PubMed
-
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532‐1542. - PubMed
-
- Armstrong SA, Kung AL, Mabon ME, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173‐183. - PubMed
-
- Williams B, Atkins A, Zhang H, et al. Cell‐based selection of internalizing fully human antagonistic antibodies directed against FLT3 for suppression of leukemia cell growth. Leukemia. 2005;19:1432‐1438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

