Closing the Gap for Electronic Short-Circuiting: Photosystem I Mixed Monolayers Enable Improved Anisotropic Electron Flow in Biophotovoltaic Devices
- PMID: 33075190
- PMCID: PMC7894356
- DOI: 10.1002/anie.202008958
Closing the Gap for Electronic Short-Circuiting: Photosystem I Mixed Monolayers Enable Improved Anisotropic Electron Flow in Biophotovoltaic Devices
Abstract
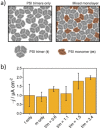
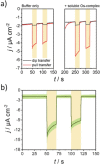
Well-defined assemblies of photosynthetic protein complexes are required for an optimal performance of semi-artificial energy conversion devices, capable of providing unidirectional electron flow when light-harvesting proteins are interfaced with electrode surfaces. We present mixed photosystem I (PSI) monolayers constituted of native cyanobacterial PSI trimers in combination with isolated PSI monomers from the same organism. The resulting compact arrangement ensures a high density of photoactive protein complexes per unit area, providing the basis to effectively minimize short-circuiting processes that typically limit the performance of PSI-based bioelectrodes. The PSI film is further interfaced with redox polymers for optimal electron transfer, enabling highly efficient light-induced photocurrent generation. Coupling of the photocathode with a [NiFeSe]-hydrogenase confirms the possibility to realize light-induced H2 evolution.
Keywords: Biophotovoltaics; Electrochemistry; Langmuir-Blodgett films; Photosystem I; Redox polymers.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Zhang J. Z., Reisner E., Nat. Rev. Chem. 2020, 4, 6;
-
- Kornienko N., Zhang J. Z., Sakimoto K. K., Yang P., Reisner E., Nat. Nanotechnol. 2018, 13, 890; - PubMed
-
- Musazade E., Voloshin R., Brady N., Mondal J., Atashova S., Zharmukhamedov S. K., Huseynova I., Ramakrishna S., Najafpour M. M., Shen J.-R., et al., J. Photochem. Photobiol. C 2018, 35, 134;
-
- Saboe P. O., Conte E., Farell M., Bazan G. C., Kumar M., Energy Environ. Sci. 2017, 10, 14;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

