Small cyclic sodium channel inhibitors
- PMID: 33075312
- PMCID: PMC9116130
- DOI: 10.1016/j.bcp.2020.114291
Small cyclic sodium channel inhibitors
Abstract
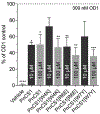
Voltage-gated sodium (NaV) channels play crucial roles in a range of (patho)physiological processes. Much interest has arisen within the pharmaceutical industry to pursue these channels as analgesic targets following overwhelming evidence that NaV channel subtypes NaV1.7-NaV1.9 are involved in nociception. More recently, NaV1.1, NaV1.3 and NaV1.6 have also been identified to be involved in pain pathways. Venom-derived disulfide-rich peptide toxins, isolated from spiders and cone snails, have been used extensively as probes to investigate these channels and have attracted much interest as drug leads. However, few peptide-based leads have made it as drugs due to unfavourable physiochemical attributes including poor in vivo pharmacokinetics and limited oral bioavailability. The present work aims to bridge the gap in the development pipeline between drug leads and drug candidates by downsizing these larger venom-derived NaV inhibitors into smaller, more "drug-like" molecules. Here, we use molecular engineering of small cyclic peptides to aid in the determination of what drives subtype selectivity and molecular interactions of these downsized inhibitors across NaV subtypes. We designed a series of small, stable and novel NaV probes displaying NaV subtype selectivity and potency in vitro coupled with potent in vivo analgesic activity, involving yet to be elucidated analgesic pathways in addition to NaV subtype modulation.
Keywords: Cone snail toxin; Cyclic peptide; Nociception; Pain; Spider toxin; Voltage gated sodium channel.
Published by Elsevier Inc.
Figures
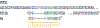
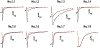
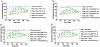

Similar articles
-
Where cone snails and spiders meet: design of small cyclic sodium-channel inhibitors.FASEB J. 2019 Mar;33(3):3693-3703. doi: 10.1096/fj.201801909R. Epub 2018 Dec 3. FASEB J. 2019. PMID: 30509130
-
Spider and scorpion knottins targeting voltage-gated sodium ion channels in pain signaling.Biochem Pharmacol. 2024 Sep;227:116465. doi: 10.1016/j.bcp.2024.116465. Epub 2024 Aug 3. Biochem Pharmacol. 2024. PMID: 39102991 Review.
-
Modulatory features of the novel spider toxin μ-TRTX-Df1a isolated from the venom of the spider Davus fasciatus.Br J Pharmacol. 2017 Aug;174(15):2528-2544. doi: 10.1111/bph.13865. Epub 2017 Jun 27. Br J Pharmacol. 2017. PMID: 28542706 Free PMC article.
-
Identification and Characterization of ProTx-III [μ-TRTX-Tp1a], a New Voltage-Gated Sodium Channel Inhibitor from Venom of the Tarantula Thrixopelma pruriens.Mol Pharmacol. 2015 Aug;88(2):291-303. doi: 10.1124/mol.115.098178. Epub 2015 May 15. Mol Pharmacol. 2015. PMID: 25979003
-
Selective Voltage-Gated Sodium Channel Peptide Toxins from Animal Venom: Pharmacological Probes and Analgesic Drug Development.ACS Chem Neurosci. 2018 Feb 21;9(2):187-197. doi: 10.1021/acschemneuro.7b00406. Epub 2017 Dec 8. ACS Chem Neurosci. 2018. PMID: 29161016 Review.
Cited by
-
Diversely evolved xibalbin variants from remipede venom inhibit potassium channels and activate PKA-II and Erk1/2 signaling.BMC Biol. 2024 Jul 29;22(1):164. doi: 10.1186/s12915-024-01955-5. BMC Biol. 2024. PMID: 39075558 Free PMC article.
-
Venom biotechnology: casting light on nature's deadliest weapons using synthetic biology.Front Bioeng Biotechnol. 2023 May 3;11:1166601. doi: 10.3389/fbioe.2023.1166601. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37207126 Free PMC article. Review.
-
Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets.Front Pain Res (Lausanne). 2021 Dec 13;2:750583. doi: 10.3389/fpain.2021.750583. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295464 Free PMC article. Review.
-
Apamin structure and pharmacology revisited.Front Pharmacol. 2022 Sep 16;13:977440. doi: 10.3389/fphar.2022.977440. eCollection 2022. Front Pharmacol. 2022. PMID: 36188602 Free PMC article.
-
Cyclic Peptides as T-Type Calcium Channel Blockers: Characterization and Molecular Mapping of the Binding Site.ACS Pharmacol Transl Sci. 2021 Jun 7;4(4):1379-1389. doi: 10.1021/acsptsci.1c00079. eCollection 2021 Aug 13. ACS Pharmacol Transl Sci. 2021. PMID: 34423272 Free PMC article.
References
-
- Robinson SD, Undheim EAB, Ueberheide B, King GF, Venom peptides as therapeutics: advances, challenges and the future of venom-peptide discovery, Expert Rev. Proteomics 14 (10) (2017) 931–939. - PubMed
-
- Prashanth JR, Dutertre S, Lewis RJ, Pharmacology of predatory and defensive venom peptides in cone snails, Mol. Biosyst 13 (12) (2017) 2453–2465. - PubMed
-
- Green BR, Olivera BM, Venom peptides from cone snails: pharmacological probes for voltage-gated sodium channels, Curr. Top. Membr 78 (2016) 65–86. - PubMed
-
- Israel MR, Tay B, Deuis JR, Vetter I, Sodium channels and venom peptide pharmacology, Adv. Pharmacol 79 (2017) 67–116. - PubMed
-
- Lewis RJ, Dutertre S, Vetter I, Christie MJ, Conus venom peptide pharmacology, Pharmacol. Rev 64 (2) (2012) 259–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

