Consensus Guidelines for Evaluation and Management of Pulmonary Disease in Sjögren's
- PMID: 33075377
- PMCID: PMC8438162
- DOI: 10.1016/j.chest.2020.10.011
Consensus Guidelines for Evaluation and Management of Pulmonary Disease in Sjögren's
Abstract
Background: Pulmonary disease is a potentially serious yet underdiagnosed complication of Sjögren's syndrome, the second most common autoimmune rheumatic disease. Approximately 16% of patients with Sjögren's demonstrate pulmonary involvement with higher mortality and lower quality of life.
Research question: Clinical practice guidelines for pulmonary manifestations of Sjögren's were developed by the Sjögren's Foundation after identifying a critical need for early diagnosis and improved quality and consistency of care.
Study design and methods: A rigorous and transparent methodology was followed according to American College of Rheumatology guidelines. The Pulmonary Topic Review Group (TRG) developed clinical questions in the PICO (Patient, Intervention, Comparison, Outcome) format and selected literature search parameters. Each article was reviewed by a minimum of two TRG members for eligibility and assessment of quality of evidence and strength of recommendation. Guidelines were then drafted based on available evidence, expert opinion, and clinical importance. Draft recommendations with a clinical rationale and data extraction tables were submitted to a Consensus Expert Panel for consideration and approval, with at least 75% agreement required for individual recommendations to be included in the final version.
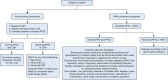
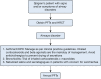
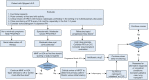
Results: The literature search revealed 1,192 articles, of which 150 qualified for consideration in guideline development. Of the original 85 PICO questions posed by the TRG, 52 recommendations were generated. These were then reviewed by the Consensus Expert Panel and 52 recommendations were finalized, with a mean agreement of 97.71% (range, 79%-100%). The recommendations span topics of evaluating Sjögren's patients for pulmonary manifestations and assessing, managing, and treating upper and lower airway disease, interstitial lung disease, and lymphoproliferative disease.
Interpretation: Clinical practice guidelines for pulmonary manifestations in Sjögren's will improve early identification, evaluation, and uniformity of care by primary care physicians, rheumatologists, and pulmonologists. Additionally, opportunities for future research are identified.
Keywords: Sjögren; Sjögren's; guideline; lung; pulmonary.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Airway Hydration in Sjögren's Pulmonary Disease: The Role of Nebulized Saline Solution Beyond Xerotrachea.Chest. 2021 Sep;160(3):e322. doi: 10.1016/j.chest.2021.04.031. Chest. 2021. PMID: 34488983 No abstract available.
-
Response.Chest. 2021 Sep;160(3):e323. doi: 10.1016/j.chest.2021.04.030. Chest. 2021. PMID: 34488985 No abstract available.
References
-
- Vivino F.B. Sjögren’s syndrome: clinical aspects. Clin Immunol. 2017;182:48–54. - PubMed
-
- Helmick C.G., Felson D.T., Lawrence R.C. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. - PubMed
-
- Whitacre C.C. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–780. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

