Design of bivalent ligands targeting putative GPCR dimers
- PMID: 33075471
- PMCID: PMC7856001
- DOI: 10.1016/j.drudis.2020.10.006
Design of bivalent ligands targeting putative GPCR dimers
Abstract
G protein-coupled receptors (GPCRs) have been exploited as primary targets for drug discovery, and GPCR dimerization offers opportunities for drug design and disease treatment. An important strategy for targeting putative GPCR dimers is the use of bivalent ligands, which are single molecules that contain two pharmacophores connected through a spacer. Here, we discuss the selection of pharmacophores, the optimal length and chemical composition of the spacer, and the choice of spacer attachment points to the pharmacophores. Furthermore, we review the most recent advances (from 2018 to the present) in the design, discovery and development of bivalent ligands. We aim to reveal the state-of-the-art design strategy for bivalent ligands and provide insights into future opportunities in this promising field of drug discovery.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no competing financial interests.
Figures



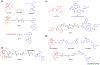
References
-
- Hazum E et al. (1979) Opiate (enkephalin) receptors of neuroblastoma cells: occurrence in clusters on the cell surface. Science 206,1077–1079 - PubMed
-
- Cvejic S and Devi LA (1997) Dimerization of the δ opioid receptor: implication for a role in receptor internalization. Journal of Biological Chemistry 272, 26959–26964 - PubMed
-
- Portoghese PS (2001) From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. Journal of Medicinal Chemistry 44, 2259–2269 - PubMed
-
- Ng GYK et al. (1996) Dopamine D2 receptor dimers and receptor-blocking peptides. Biochemical and Biophysical Research Communications 227, 200–204 - PubMed
-
- Fiorentini C et al. (2010) Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Current Opinion in Pharmacology 10, 87–92 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

