Methylene blue inhibits replication of SARS-CoV-2 in vitro
- PMID: 33075512
- PMCID: PMC7566888
- DOI: 10.1016/j.ijantimicag.2020.106202
Methylene blue inhibits replication of SARS-CoV-2 in vitro
Abstract
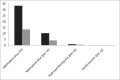
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus diseases 2019 (COVID-19) emerged in Wuhan, China. Currently there is no antiviral treatment recommended against SARS-CoV-2. Identifying effective antiviral drugs is urgently required. Methylene blue has already demonstrated in vitro antiviral activity in photodynamic therapy as well as antibacterial, antifungal and antiparasitic activities in non-photodynamic assays. In this study. non-photoactivated methylene blue showed in vitro activity at very low micromolar range with an EC50 (median effective concentration) of 0.30 ± 0.03 μM and an EC90 (90% effective concentration) of 0.75 ± 0.21 μM at a multiplicity of infection (MOI) of 0.25 against SARS-CoV-2 (strain IHUMI-3). The EC50 and EC90 values for methylene blue are lower than those obtained for hydroxychloroquine (1.5 μM and 3.0 μM) and azithromycin (20.1 μM and 41.9 μM). The ratios Cmax/EC50 and Cmax/EC90 in blood for methylene blue were estimated at 10.1 and 4.0, respectively, following oral administration and 33.3 and 13.3 following intravenous administration. Methylene blue EC50 and EC90 values are consistent with concentrations observed in human blood. We propose that methylene blue is a promising drug for treatment of COVID-19. In vivo evaluation in animal experimental models is now required to confirm its antiviral effects on SARS-CoV-2. The potential interest of methylene blue to treat COVID-19 needs to be confirmed by prospective comparative clinical studies.
Keywords: Antiviral; COVID-19; Coronavirus; In vitro; Methylene blue; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- Fryk JJ, Marks DC, Hobson-Peters J, Prow NA, Watterson D, Hall RA, et al. Dengue and chikungunya viruses in plasma are effectively inactivated after treatment with methylene blue and visible light. Transfusion. 2016;56:2278–2285. - PubMed
-
- Faddy HM, Fryk JJ, Hal RA, Young PR, Reichenberg S, Tolksdorf F, et al. Inactivation of yellow fever virus in plasma after treatment with methylene blue and visible light and in platelet concentrates following treatment with ultraviolet C light. Transfusion. 2019;59:2223–2227. - PubMed
-
- Wang Y, Ren K, Liao X, Luo G, Kumthip K, Leetrakool N, et al. Inactivation of Zika virus in plasma and derivatives by four different methods. J Med Virol. 2019;91:2059–2065. - PubMed
-
- Eickmann M, Gravemann U, Handke W, Tolksdorf F, Reichenberg S, Müller TH, et al. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion. 2018;58:2202–2207. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

