Establishment of Acquired Cisplatin Resistance in Ovarian Cancer Cell Lines Characterized by Enriched Metastatic Properties with Increased Twist Expression
- PMID: 33076245
- PMCID: PMC7589258
- DOI: 10.3390/ijms21207613
Establishment of Acquired Cisplatin Resistance in Ovarian Cancer Cell Lines Characterized by Enriched Metastatic Properties with Increased Twist Expression
Abstract
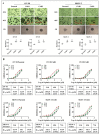
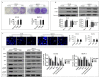
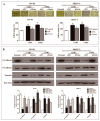
Ovarian cancer (OC) is the most lethal of the gynecologic cancers, and platinum-based treatment is a part of the standard first-line chemotherapy regimen. However, rapid development of acquired cisplatin resistance remains the main cause of treatment failure, and the underlying mechanism of resistance in OC treatment remains poorly understood. Faced with this problem, our aim in this study was to generate cisplatin-resistant (CisR) OC cell models in vitro and investigate the role of epithelial-mesenchymal transition (EMT) transcription factor Twist on acquired cisplatin resistance in OC cell models. To achieve this aim, OC cell lines OV-90 and SKOV-3 were exposed to cisplatin using pulse dosing and stepwise dose escalation methods for a duration of eight months, and a total of four CisR sublines were generated, two for each cell line. The acquired cisplatin resistance was confirmed by determination of 50% inhibitory concentration (IC50) and clonogenic survival assay. Furthermore, the CisR cells were studied to assess their respective characteristics of metastasis, EMT phenotype, DNA repair and endoplasmic reticulum stress-mediated cell death. We found the IC50 of CisR cells to cisplatin was 3-5 times higher than parental cells. The expression of Twist and metastatic ability of CisR cells were significantly greater than those of sensitive cells. The CisR cells displayed an EMT phenotype with decreased epithelial cell marker E-cadherin and increased mesenchymal proteins N-cadherin and vimentin. We observed that CisR cells showed significantly higher expression of DNA repair proteins, X-ray repair cross-complementing protein 1 (XRCC1) and poly (ADP-ribose) polymerases 1 (PARP1), with significantly reduced endoplasmic reticulum (ER) stress-mediated cell death. Moreover, Twist knockdown reduced metastatic ability of CisR cells by suppressing EMT, DNA repair and inducing ER stress-induced cell death. In conclusion, we highlighted the utilization of an acquired cisplatin resistance model to identify the potential role of Twist as a therapeutic target to reverse acquired cisplatin resistance in OC.
Keywords: Twist; cisplatin resistance; epithelial–mesenchymal transition; metastasis; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

