Molecular Mechanisms and Emerging Therapeutics for Osteoporosis
- PMID: 33076329
- PMCID: PMC7589419
- DOI: 10.3390/ijms21207623
Molecular Mechanisms and Emerging Therapeutics for Osteoporosis
Abstract
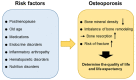
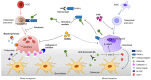
Osteoporosis is the most common chronic metabolic bone disease. It has been estimated that more than 10 million people in the United States and 200 million men and women worldwide have osteoporosis. Given that the aging population is rapidly increasing in many countries, osteoporosis could become a global challenge with an impact on the quality of life of the affected individuals. Osteoporosis can be defined as a condition characterized by low bone density and increased risk of fractures due to the deterioration of the bone architecture. Thus, the major goal of treatment is to reduce the risk for fractures. There are several treatment options, mostly medications that can control disease progression in risk groups, such as postmenopausal women and elderly men. Recent studies on the basic molecular mechanisms and clinical implications of osteoporosis have identified novel therapeutic targets. Emerging therapies targeting novel disease mechanisms could provide powerful approaches for osteoporosis management in the future. Here, we review the etiology of osteoporosis and the molecular mechanism of bone remodeling, present current pharmacological options, and discuss emerging therapies targeting novel mechanisms, investigational treatments, and new promising therapeutic approaches.
Keywords: fracture; medication; molecular mechanism; novel approach; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wright N.C., Looker A.C., Saag K.G., Curtis J.R., Delzell E.S., Randall S., Dawson--Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone. Min. Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

