Vasoconstrictor Mechanisms in Chronic Hypoxia-Induced Pulmonary Hypertension: Role of Oxidant Signaling
- PMID: 33076504
- PMCID: PMC7602539
- DOI: 10.3390/antiox9100999
Vasoconstrictor Mechanisms in Chronic Hypoxia-Induced Pulmonary Hypertension: Role of Oxidant Signaling
Abstract
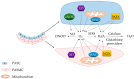
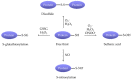
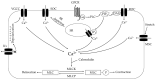
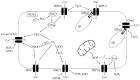
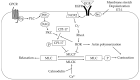
Elevated resistance of pulmonary circulation after chronic hypoxia exposure leads to pulmonary hypertension. Contributing to this pathological process is enhanced pulmonary vasoconstriction through both calcium-dependent and calcium sensitization mechanisms. Reactive oxygen species (ROS), as a result of increased enzymatic production and/or decreased scavenging, participate in augmentation of pulmonary arterial constriction by potentiating calcium influx as well as activation of myofilament sensitization, therefore mediating the development of pulmonary hypertension. Here, we review the effects of chronic hypoxia on sources of ROS within the pulmonary vasculature including NADPH oxidases, mitochondria, uncoupled endothelial nitric oxide synthase, xanthine oxidase, monoamine oxidases and dysfunctional superoxide dismutases. We also summarize the ROS-induced functional alterations of various Ca2+ and K+ channels involved in regulating Ca2+ influx, and of Rho kinase that is responsible for myofilament Ca2+ sensitivity. A variety of antioxidants have been shown to have beneficial therapeutic effects in animal models of pulmonary hypertension, supporting the role of ROS in the development of pulmonary hypertension. A better understanding of the mechanisms by which ROS enhance vasoconstriction will be useful in evaluating the efficacy of antioxidants for the treatment of pulmonary hypertension.
Keywords: calcium influx; calcium sensitization; chronic hypoxia; pulmonary hypertension; pulmonary vasoconstriction; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Brown L.M., Chen H., Halpern S., Taichman D., McGoon M.D., Farber H.W., Frost A.E., Liou T.G., Turner M., Feldkircher K., et al. Delay in recognition of pulmonary arterial hypertension: Factors identified from the REVEAL Registry. Chest. 2011;140:19–26. doi: 10.1378/chest.10-1166. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

