Nonhistone Proteins HMGB1 and HMGB2 Differentially Modulate the Response of Human Embryonic Stem Cells and the Progenitor Cells to the Anticancer Drug Etoposide
- PMID: 33076532
- PMCID: PMC7602880
- DOI: 10.3390/biom10101450
Nonhistone Proteins HMGB1 and HMGB2 Differentially Modulate the Response of Human Embryonic Stem Cells and the Progenitor Cells to the Anticancer Drug Etoposide
Abstract
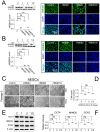
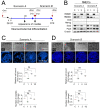
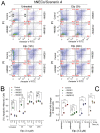
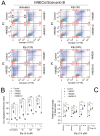
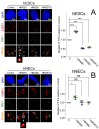
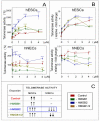
HMGB1 and HMGB2 proteins are abundantly expressed in human embryonic stem cells (hESCs) and hESC-derived progenitor cells (neuroectodermal cells, hNECs), though their functional roles in pluripotency and the mechanisms underlying their differentiation in response to the anticancer drug etoposide remain to be elucidated. Here, we show that HMGB1 and/or HMGB2 knockdown (KD) by shRNA in hESCs did not affect the cell stemness/pluripotency regardless of etoposide treatments, while in hESC-derived neuroectodermal cells, treatment resulted in differential effects on cell survival and the generation of rosette structures. The objective of this work was to determine whether HMGB1/2 proteins could modulate the sensitivity of hESCs and hESC-derived progenitor cells (hNECs) to etoposide. We observed that HMGB1 KD knockdown (KD) and, to a lesser extent, HMGB2 KD enhanced the sensitivity of hESCs to etoposide. Enhanced accumulation of 53BP1 on telomeres was detected by confocal microscopy in both untreated and etoposide-treated HMGB1 KD hESCs and hNECs, indicating that the loss of HMGB1 could destabilize telomeres. On the other hand, decreased accumulation of 53BP1 on telomeres in etoposide-treated HMGB2 KD hESCs (but not in HMGB2 KD hNECs) suggested that the loss of HMGB2 promoted the stability of telomeres. Etoposide treatment of hESCs resulted in a significant enhancement of telomerase activity, with the highest increase observed in the HMGB2 KD cells. Interestingly, no changes in telomerase activity were found in etoposide-treated control hNECs, but HMGB2 KD (unlike HMGB1 KD) markedly decreased telomerase activity in these cells. Changes in telomerase activity in the etoposide-treated HMGB2 KD hESCs or hNECs coincided with the appearance of DNA damage markers and could already be observed before the onset of apoptosis. Collectively, we have demonstrated that HMGB1 or HMGB2 differentially modulate the impact of etoposide treatment on human embryonic stem cells and their progenitor cells, suggesting possible strategies for the enhancement of the efficacy of this anticancer drug.
Keywords: HMGB1 and HMGB2; apoptosis; etoposide; human embryonic stem cells; neuroectodermal cells; telomerase.
Conflict of interest statement
The authors declare no conflict of interest
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

