Imaging Cardiovascular and Lung Macrophages With the Positron Emission Tomography Sensor 64Cu-Macrin in Mice, Rabbits, and Pigs
- PMID: 33076700
- PMCID: PMC7583675
- DOI: 10.1161/CIRCIMAGING.120.010586
Imaging Cardiovascular and Lung Macrophages With the Positron Emission Tomography Sensor 64Cu-Macrin in Mice, Rabbits, and Pigs
Abstract
Background: Macrophages, innate immune cells that reside in all organs, defend the host against infection and injury. In the heart and vasculature, inflammatory macrophages also enhance tissue damage and propel cardiovascular diseases.
Methods: We here use in vivo positron emission tomography (PET) imaging, flow cytometry, and confocal microscopy to evaluate quantitative noninvasive assessment of cardiac, arterial, and pulmonary macrophages using the nanotracer 64Cu-Macrin-a 20-nm spherical dextran nanoparticle assembled from nontoxic polyglucose.
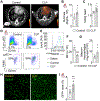
Results: PET imaging using 64Cu-Macrin faithfully reported accumulation of macrophages in the heart and lung of mice with myocardial infarction, sepsis, or pneumonia. Flow cytometry and confocal microscopy detected the near-infrared fluorescent version of the nanoparticle (VT680Macrin) primarily in tissue macrophages. In 5-day-old mice, 64Cu-Macrin PET imaging quantified physiologically more numerous cardiac macrophages. Upon intravenous administration of 64Cu-Macrin in rabbits and pigs, we detected heightened macrophage numbers in the infarcted myocardium, inflamed lung regions, and atherosclerotic plaques using a clinical PET/magnetic resonance imaging scanner. Toxicity studies in rats and human dosimetry estimates suggest that 64Cu-Macrin is safe for use in humans.
Conclusions: Taken together, these results indicate 64Cu-Macrin could serve as a facile PET nanotracer to survey spatiotemporal macrophage dynamics during various physiological and pathological conditions. 64Cu-Macrin PET imaging could stage inflammatory cardiovascular disease activity, assist disease management, and serve as an imaging biomarker for emerging macrophage-targeted therapeutics.
Keywords: heart; macrophages; myocardial infarction; nanoparticles; pneumonia.
Figures




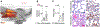


Comment in
-
Delineating the Role of Macrophages in Cardiovascular Disease: How Specific Do We Need to Be?Circ Cardiovasc Imaging. 2020 Oct;13(10):e011605. doi: 10.1161/CIRCIMAGING.120.011605. Epub 2020 Oct 20. Circ Cardiovasc Imaging. 2020. PMID: 33076697 Free PMC article. No abstract available.
References
-
- Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

