Gut bacterial deamination of residual levodopa medication for Parkinson's disease
- PMID: 33076930
- PMCID: PMC7574542
- DOI: 10.1186/s12915-020-00876-3
Gut bacterial deamination of residual levodopa medication for Parkinson's disease
Abstract
Background: Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by both motor and non-motor symptoms. Gastrointestinal tract dysfunction is one of the non-motor features, where constipation is reported as the most common gastrointestinal symptom. Aromatic bacterial metabolites are attracting considerable attention due to their impact on gut homeostasis and host's physiology. In particular, Clostridium sporogenes is a key contributor to the production of these bioactive metabolites in the human gut.
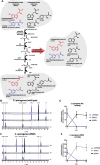
Results: Here, we show that C. sporogenes deaminates levodopa, the main treatment in Parkinson's disease, and identify the aromatic aminotransferase responsible for the initiation of the deamination pathway. The deaminated metabolite from levodopa, 3-(3,4-dihydroxyphenyl)propionic acid, elicits an inhibitory effect on ileal motility in an ex vivo model. We detected 3-(3,4-dihydroxyphenyl)propionic acid in fecal samples of Parkinson's disease patients on levodopa medication and found that this metabolite is actively produced by the gut microbiota in those stool samples.
Conclusions: Levodopa is deaminated by the gut bacterium C. sporogenes producing a metabolite that inhibits ileal motility ex vivo. Overall, this study underpins the importance of the metabolic pathways of the gut microbiome involved in drug metabolism not only to preserve drug effectiveness, but also to avoid potential side effects of bacterial breakdown products of the unabsorbed residue of medication.
Keywords: Aminotransferase; Bioactive metabolites; Clostridium sporogenes; Drug side effects; Gastrointestinal motility; Non-motor symptoms.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

