Pertussis vaccination in pregnancy in Canada: a cost-utility analysis
- PMID: 33077536
- PMCID: PMC7588263
- DOI: 10.9778/cmajo.20200060
Pertussis vaccination in pregnancy in Canada: a cost-utility analysis
Abstract
Background: The Canadian National Advisory Committee on Immunization recommends universal vaccination against pertussis in pregnancy. We assessed the cost-effectiveness of vaccination with tetanus-diphtheria-acellular pertussis (Tdap) vaccine in pregnancy in Canada.
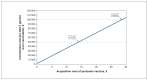
Methods: We conducted a cost-utility analysis comparing a vaccination program to no program corresponding with the 2017 Canadian guideline for economic evaluation from the Canadian Agency for Drugs and Technologies in Health. We developed 2 models - part decision tree, part Markov model - to estimate the long-term cost and quality-adjusted life-years (QALYs) for pregnant women and their infants. We obtained epidemiologic data from 2006 to 2015, and derived costs and utility values from relevant sources. Results were reported in 2019 Canadian dollars. We obtained expected values through probabilistic analysis, with methodologic and structural uncertainty assessed through scenario analyses. The analysis adopted an acquisition price of Tdap vaccine of $12.50, with scenario analysis conducted to identify the threshold price for vaccination to be cost-effective.
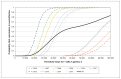
Results: In the base-case scenario, for every 1000 pregnant women vaccinated, the program would lead to a gain of 0.3 QALYs, occurring solely in infants, at an increased total cost of $12 987, or $44 301 per QALY gained. Based on a threshold of $50 000 per QALY gained, vaccination would have been cost-effective in 6 of the 10 years included in the model (range of incremental costs $20 463-$100 348 per QALY gained). The threshold cost for Tdap vaccine to be cost-effective over the 10-year horizon was $14.03.
Interpretation: Based on a threshold of $50 000 per QALY gained, vaccination against pertussis in pregnancy would be cost-effective if the acquisition cost per vaccine were $14.03 or less. Province- and territory-specific analyses should be done to inform local decision-making.
Copyright 2020, Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Manish Sadarangani is supported via salary awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research. He has been an investigator on projects funded by Merck, VBI Vaccines, Pfizer, Seqirus, Sanofi Pasteur and GlaxoSmithKline; all funds have been paid to his institute, and he has not received any personal payments. Scott Halperin has been an investigator on projects funded by Merck, VBI Vaccines, Pfizer, Seqirus, Sanofi Pasteur and GlaxoSmithKline, and has served on ad hoc advisory boards for Merck, Sanofi Pasteur, GlaxoSmithKline and Pfizer; all funds have been paid to his university, and he has not received any personal payments. No other competing interests were declared.
Figures

References
-
- Yeung KHT, Duclos P, Nelson EAS, et al. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17:974–80. - PubMed
-
- Bettinger JA, Halperin SA, De Serres G, et al. The effect of changing from whole-cell to acellular pertussis vaccine on the epidemiology of hospitalized children with pertussis in Canada. Pediatr Infect Dis J. 2007;26:31–5. - PubMed
-
- Vaccine preventable disease: surveillance report to December 31, 2015. Ottawa: Public Health Agency of Canada; 2017.
-
- Pertussis epidemic — Washington, 2012. MMWR Morb Mortal Wkly Rep. (Centers for Disease Control and Prevention (CDC)) 2012;61:517–22. - PubMed
-
- Winter K, Harriman K, Zipprich J, et al. California pertussis epidemic, 2010. J Pediatr. 2012;161:1091–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
