Liquid-liquid phase separation promotes animal desiccation tolerance
- PMID: 33077592
- PMCID: PMC7959570
- DOI: 10.1073/pnas.2014463117
Liquid-liquid phase separation promotes animal desiccation tolerance
Abstract
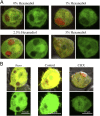
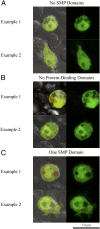
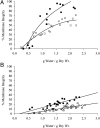
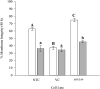
Proteinaceous liquid-liquid phase separation (LLPS) occurs when a polypeptide coalesces into a dense phase to form a liquid droplet (i.e., condensate) in aqueous solution. In vivo, functional protein-based condensates are often referred to as membraneless organelles (MLOs), which have roles in cellular processes ranging from stress responses to regulation of gene expression. Late embryogenesis abundant (LEA) proteins containing seed maturation protein domains (SMP; PF04927) have been linked to storage tolerance of orthodox seeds. The mechanism by which anhydrobiotic longevity is improved is unknown. Interestingly, the brine shrimp Artemia franciscana is the only animal known to express such a protein (AfrLEA6) in its anhydrobiotic embryos. Ectopic expression of AfrLEA6 (AWM11684) in insect cells improves their desiccation tolerance and a fraction of the protein is sequestered into MLOs, while aqueous AfrLEA6 raises the viscosity of the cytoplasm. LLPS of AfrLEA6 is driven by the SMP domain, while the size of formed MLOs is regulated by a domain predicted to engage in protein binding. AfrLEA6 condensates formed in vitro selectively incorporate target proteins based on their surface charge, while cytoplasmic MLOs formed in AfrLEA6-transfected insect cells behave like stress granules. We suggest that AfrLEA6 promotes desiccation tolerance by engaging in two distinct molecular mechanisms: by raising cytoplasmic viscosity at even modest levels of water loss to promote cell integrity during drying and by forming condensates that may act as protective compartments for desiccation-sensitive proteins. Identifying and understanding the molecular mechanisms that govern anhydrobiosis will lead to significant advancements in preserving biological samples.
Keywords: cryptobiosis; late embryogenesis abundant; liquid-liquid phase separation; membraneless organelle; water stress.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Benner S. A., Ricardo A., Carrigan M. A., Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 8, 672–689 (2004). - PubMed
-
- Yancey P. H., Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208, 2819–2830 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

