An Exposure-Response Perspective on the Clinical Dose of Pretomanid
- PMID: 33077660
- PMCID: PMC7927811
- DOI: 10.1128/AAC.01121-20
An Exposure-Response Perspective on the Clinical Dose of Pretomanid
Abstract
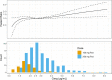
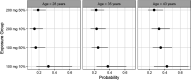
Pretomanid was approved by the U.S. FDA, via the limited population pathway for antibacterial and antifungal drugs, as part of a three-drug regimen with bedaquiline and linezolid for the treatment of extensively drug-resistant and treatment-intolerant or nonresponsive multidrug-resistant tuberculosis. The recommended dose of pretomanid is 200 mg once daily with food. The objective of this work was to retrospectively evaluate this recommended dose by means of exposure-response (E-R) modeling applied to outcomes of both efficacy and safety. Cox proportional-hazards modeling was used, with the steady-state average pretomanid concentration as the exposure metric. The efficacy outcome was time to sputum culture conversion (TSCC) to negative. The safety outcomes were times to the first occurrence of adverse events in classes selected from either pretomanid's investigator brochure or the new drug application (NDA) submission as recognized safety signals for pretomanid based on preclinical as well as clinical experience. Significant E-R relationships were found for TSCC and two adverse-event classes, vomiting (a single preferred term) and gastrointestinal (GI) symptoms (a collection of related terms). No significant E-R relationships were found for the single preferred terms nausea, alanine aminotransferase (ALT) increased, aspartate aminotransferase (AST) increased, and headache and for the collections hepatic disorders, transaminases increased, skin and subcutaneous tissue disorders, and headache. The results suggest that the recommended dose of pretomanid, 200 mg given in the fed state, is appropriate over the range of pharmacokinetic exposures.
Keywords: antimicrobial agents; pretomanid; tuberculosis.
Copyright © 2020 Nedelman et al.
Figures


References
-
- Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, for the Nix-TB Trial Team. 2020. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 382:893–902. doi:10.1056/NEJMoa1901814. - DOI - PMC - PubMed
-
- Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother 54:3402–3407. doi:10.1128/AAC.01354-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

