Forebrain delta opioid receptors regulate the response of delta agonist in models of migraine and opioid-induced hyperalgesia
- PMID: 33077757
- PMCID: PMC7573615
- DOI: 10.1038/s41598-020-74605-9
Forebrain delta opioid receptors regulate the response of delta agonist in models of migraine and opioid-induced hyperalgesia
Abstract
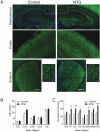
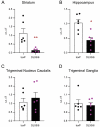
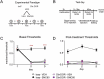
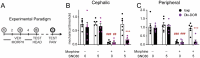
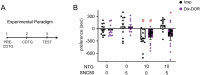
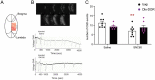
Delta opioid receptor (DOR) agonists have been identified as a promising novel therapy for headache disorders. DORs are broadly expressed in several peripheral and central regions important for pain processing and mood regulation; and it is unclear which receptors regulate headache associated symptoms. In a model of chronic migraine-associated pain using the human migraine trigger, nitroglycerin, we observed increased expression of DOR in cortex, hippocampus, and striatum; suggesting a role for these forebrain regions in the regulation of migraine. To test this hypothesis, we used conditional knockout mice with DORs deleted from forebrain GABAergic neurons (Dlx-DOR), and investigated the outcome of this knockout on the effectiveness of the DOR agonist SNC80 in multiple headache models. In DOR loxP controls SNC80 blocked the development of acute and chronic cephalic allodynia in the chronic nitroglycerin model, an effect that was lost in Dlx-DOR mice. In addition, the anti-allodynic effects of SNC80 were lost in a model of opioid induced hyperalgesia/medication overuse headache in Dlx-DOR conditional knockouts. In a model reflecting negative affect associated with migraine, SNC80 was only effective in loxP controls and not Dlx-DOR mice. Similarly, SNC80 was ineffective in the cortical spreading depression model of migraine aura in conditional knockout mice. Taken together, these data indicate that forebrain DORs are necessary for the action of DOR agonists in relieving headache-related symptoms and suggest that forebrain regions may play an important role in migraine modulation.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures.Behav Brain Res. 2015 Feb 1;278:429-34. doi: 10.1016/j.bbr.2014.10.029. Epub 2014 Oct 30. Behav Brain Res. 2015. PMID: 25447299 Free PMC article.
-
δ-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice.Br J Pharmacol. 2014 May;171(9):2375-84. doi: 10.1111/bph.12591. Br J Pharmacol. 2014. PMID: 24467301 Free PMC article.
-
A novel anxiogenic role for the delta opioid receptor expressed in GABAergic forebrain neurons.Biol Psychiatry. 2015 Feb 15;77(4):404-15. doi: 10.1016/j.biopsych.2014.07.033. Epub 2014 Aug 27. Biol Psychiatry. 2015. PMID: 25444168 Free PMC article.
-
Alleviating pain with delta opioid receptor agonists: evidence from experimental models.J Neural Transm (Vienna). 2020 Apr;127(4):661-672. doi: 10.1007/s00702-020-02172-4. Epub 2020 Mar 18. J Neural Transm (Vienna). 2020. PMID: 32189076 Review.
-
Delta opioid receptors: Overlooked outlier or the next big thing.Curr Opin Pharmacol. 2025 Aug;83:102528. doi: 10.1016/j.coph.2025.102528. Epub 2025 May 8. Curr Opin Pharmacol. 2025. PMID: 40450836 Review.
Cited by
-
Efficacy of dual enkephalinase inhibition in a preclinical migraine model is mediated by activation of peripheral delta opioid receptors.Headache. 2023 May;63(5):621-633. doi: 10.1111/head.14517. Headache. 2023. PMID: 37183526 Free PMC article.
-
Alternative Splicing Mechanisms Underlying Opioid-Induced Hyperalgesia.Genes (Basel). 2021 Oct 1;12(10):1570. doi: 10.3390/genes12101570. Genes (Basel). 2021. PMID: 34680965 Free PMC article.
-
Delta opioid receptors in Nav1.8 expressing peripheral neurons partially regulate the effect of delta agonist in models of migraine and opioid-induced hyperalgesia.Neurobiol Pain. 2022 Jul 11;12:100099. doi: 10.1016/j.ynpai.2022.100099. eCollection 2022 Aug-Dec. Neurobiol Pain. 2022. PMID: 35859654 Free PMC article.
-
Neuronal complexity is attenuated in preclinical models of migraine and restored by HDAC6 inhibition.Elife. 2021 Apr 15;10:e63076. doi: 10.7554/eLife.63076. Elife. 2021. PMID: 33856345 Free PMC article.
-
The expression of delta opioid receptor mRNA in adult male zebra finches (Taenopygia guttata).PLoS One. 2021 Aug 31;16(8):e0256599. doi: 10.1371/journal.pone.0256599. eCollection 2021. PLoS One. 2021. PMID: 34464410 Free PMC article.
References
-
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia38, 1–211, 10.1177/0333102417738202 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

