Heat-responsive and time-resolved transcriptome and metabolome analyses of Escherichia coli uncover thermo-tolerant mechanisms
- PMID: 33077799
- PMCID: PMC7572479
- DOI: 10.1038/s41598-020-74606-8
Heat-responsive and time-resolved transcriptome and metabolome analyses of Escherichia coli uncover thermo-tolerant mechanisms
Abstract
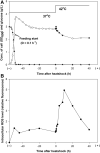
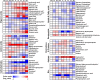
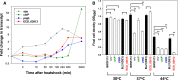
Current understanding of heat shock response has been complicated by the fact that heat stress is inevitably accompanied by changes in specific growth rates and growth stages. In this study, a chemostat culture was successfully performed to avoid the physico-chemical and biological changes that accompany heatshock, which provided a unique opportunity to investigate the full range of cellular responses to thermal stress, ranging from temporary adjustment to phenotypic adaptation at multi-omics levels. Heat-responsive and time-resolved changes in the transcriptome and metabolome of a widely used E. coli strain BL21(DE3) were explored in which the temperature was upshifted from 37 to 42 °C. Omics profiles were categorized into early (2 and 10 min), middle (0.5, 1, and 2 h), and late (4, 8, and 40 h) stages of heat stress, each of which reflected the initiation, adaptation, and phenotypic plasticity steps of the stress response. The continued heat stress modulated global gene expression by controlling the expression levels of sigma factors in different time frames, including unexpected downregulation of the second heatshock sigma factor gene (rpoE) upon the heat stress. Trehalose, cadaverine, and enterobactin showed increased production to deal with the heat-induced oxidative stress. Genes highly expressed at the late stage were experimentally validated to provide thermotolerance. Intriguingly, a cryptic capsular gene cluster showed considerably high expression level only at the late stage, and its expression was essential for cell growth at high temperature. Granule-forming and elongated cells were observed at the late stage, which was morphological plasticity occurred as a result of acclimation to the continued heat stress. Whole process of thermal adaptation along with the genetic and metabolic changes at fine temporal resolution will contribute to far-reaching comprehension of the heat shock response. Further, the identified thermotolerant genes will be useful to rationally engineer thermotolerant microorganisms.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wick LM, Egli T. Molecular components of physiological stress responses in Escherichia coli. Adv. Biochem. Eng. Biotechnol. 2004;89:1–45. - PubMed
-
- Gross, C. A. Function and regulation of the heat shock proteins in Escherichia coli and Salmonella (ed. Neidhardt, F. C.) 1382–1399 (ASM Press, London, 1996).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

