Hydrostatic pressure can induce apoptosis of the skin
- PMID: 33077833
- PMCID: PMC7572420
- DOI: 10.1038/s41598-020-74695-5
Hydrostatic pressure can induce apoptosis of the skin
Abstract
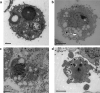
We previously showed that high hydrostatic pressure (HHP) treatment at 200 MPa for 10 min induced complete cell death in skin and skin tumors via necrosis. We used this technique to treat a giant congenital melanocytic nevus and reused the inactivated nevus tissue as a dermis autograft. However, skin inactivated by HHP promoted inflammation in a preclinical study using a porcine model. Therefore, in the present study, we explored the pressurization conditions that induce apoptosis of the skin, as apoptotic cells are not believed to promote inflammation, so the engraftment of inactivated skin should be improved. Using a human dermal fibroblast cell line in suspension culture, we found that HHP at 50 MPa for ≥ 36 h completely induced fibroblast cell death via apoptosis based on the morphological changes in transmission electron microscopy, reactive oxygen species elevation, caspase activation and phosphatidylserine membrane translocation. Furthermore, immunohistochemistry with terminal deoxynucleotidyl transferase dUTP nick-end labeling and cleaved caspase-3 showed most cells in the skin inactivated by pressurization to be apoptotic. Consequently, in vivo grafting of apoptosis-induced inactivated skin resulted in successful engraftment and greater dermal cellular density and macrophage infiltration than our existing method. Our finding supports an alternative approach to hydrostatic pressure application.
Conflict of interest statement
The authors declare no competing interests.
Figures

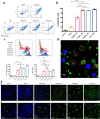
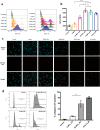
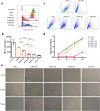
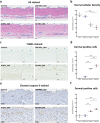
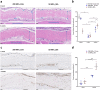
 : dermis layer. (b) The diagram comparing the dermal cellular density shows a reduction in the numbers of cell nuclei in both groups at 1 week after implantation (n = 6, p < 0.01). At week 4, more cells had infiltrated into the inactivated dermis, followed by an increase in the dermal cellular density (p < 0.0001), and the density in the dermis of the 50 MPa group was higher than that is the 200 MPa group (p < 0.05). (c) Representative anti-F4/80 immunohistochemically stained micrographs of skin grafts treated by HHP at 200 MPa for 10 min or 50 MPa for 36 h in implantation. Magnification 10×, scale bar 100 μm, (*) indicates granulation tissue. The higher-magnification micrographs in the lower right show more detail concerning each positively stained cell (brown signal); scale bar 100 μm. (d) The diagram comparing the increase in numbers of infiltrated dermal macrophages at 4 weeks after implantation in both groups (n = 6, p < 0.0001) while the number is significantly higher in the grafts of the 50 MPa group than that in the 200 MPa group (p < 0.0001).
: dermis layer. (b) The diagram comparing the dermal cellular density shows a reduction in the numbers of cell nuclei in both groups at 1 week after implantation (n = 6, p < 0.01). At week 4, more cells had infiltrated into the inactivated dermis, followed by an increase in the dermal cellular density (p < 0.0001), and the density in the dermis of the 50 MPa group was higher than that is the 200 MPa group (p < 0.05). (c) Representative anti-F4/80 immunohistochemically stained micrographs of skin grafts treated by HHP at 200 MPa for 10 min or 50 MPa for 36 h in implantation. Magnification 10×, scale bar 100 μm, (*) indicates granulation tissue. The higher-magnification micrographs in the lower right show more detail concerning each positively stained cell (brown signal); scale bar 100 μm. (d) The diagram comparing the increase in numbers of infiltrated dermal macrophages at 4 weeks after implantation in both groups (n = 6, p < 0.0001) while the number is significantly higher in the grafts of the 50 MPa group than that in the 200 MPa group (p < 0.0001).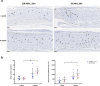
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

