Quantifying the distribution of protein oligomerization degree reflects cellular information capacity
- PMID: 33077848
- PMCID: PMC7573690
- DOI: 10.1038/s41598-020-74811-5
Quantifying the distribution of protein oligomerization degree reflects cellular information capacity
Erratum in
-
Author Correction: Quantifying the distribution of protein oligomerization degree reflects cellular information capacity.Sci Rep. 2021 Mar 26;11(1):7357. doi: 10.1038/s41598-021-86894-9. Sci Rep. 2021. PMID: 33772210 Free PMC article. No abstract available.
Abstract
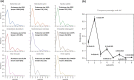
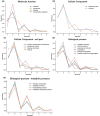
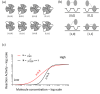
The generation of information, energy and biomass in living cells involves integrated processes that optimally evolve into complex and robust cellular networks. Protein homo-oligomerization, which is correlated with cooperativity in biology, is one means of scaling the complexity of protein networks. It can play critical roles in determining the sensitivity of genetic regulatory circuits and metabolic pathways. Therefore, understanding the roles of oligomerization may lead to new approaches of probing biological functions. Here, we analyzed the frequency of protein oligomerization degree in the cell proteome of nine different organisms, and then, we asked whether there are design trade-offs between protein oligomerization, information precision and energy costs of protein synthesis. Our results indicate that there is an upper limit for the degree of protein oligomerization, possibly because of the trade-off between cellular resource limitations and the information precision involved in biochemical reaction networks. These findings can explain the principles of cellular architecture design and provide a quantitative tool to scale synthetic biological systems.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- De Las Rivas J, Fontanillo C. Protein-protein interaction networks: Unraveling the wiring of molecular machines within the cell. Brief. Funct. Genom. 2012;11:489–496. - PubMed
-
- Costa LDF, Rodrigues FA, Cristino AS. Complex networks: The key to systems biology. Genet. Mol. Biol. 2008;31:591–601.
-
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

