Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing
- PMID: 33077938
- PMCID: PMC7885272
- DOI: 10.1038/s41551-020-00632-6
Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing
Erratum in
-
Publisher Correction: Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing.Nat Biomed Eng. 2020 Nov;4(11):1119. doi: 10.1038/s41551-020-00652-2. Nat Biomed Eng. 2020. PMID: 33122854
Abstract
Cytosine base editors and adenine base editors (ABEs) can correct point mutations predictably and independent of Cas9-induced double-stranded DNA breaks (which causes substantial indel formation) and homology-directed repair (which typically leads to low editing efficiency). Here, we show, in adult mice, that a subretinal injection of a lentivirus expressing an ABE and a single-guide RNA targeting a de novo nonsense mutation in the Rpe65 gene corrects the pathogenic mutation with up to 29% efficiency and with minimal formation of indel and off-target mutations, despite the absence of the canonical NGG sequence as a protospacer-adjacent motif. The ABE-treated mice displayed restored RPE65 expression and retinoid isomerase activity, and near-normal levels of retinal and visual functions. Our findings motivate the further testing of ABEs for the treatment of inherited retinal diseases and for the correction of pathological mutations with non-canonical protospacer-adjacent motifs.
Conflict of interest statement
Competing interests
D.R.L. is a consultant and co-founder of Beam Therapeutics, Prime Medicine, Editas Medicine and Pairwise Plants, companies that use genome editing.
Figures

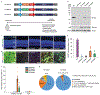


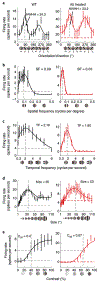
Comment in
-
Restoring vision loss with base editing.Nat Rev Drug Discov. 2020 Dec;19(12):835. doi: 10.1038/d41573-020-00186-x. Nat Rev Drug Discov. 2020. PMID: 33106643 No abstract available.
References
-
- Cremers FP, van den Hurk JA & den Hollander AI Molecular genetics of Leber congenital amaurosis. Hum. Mol. Genet. 11, 1169–1176 (2002). - PubMed
-
- Den Hollander AI, Roepman R, Koenekoop RK & Cremers FPM Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 27, 391–419 (2008). - PubMed
-
- Miraldi Utz V, Coussa RG, Antaki F & Traboulsi EI Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 39, 671–677 (2018). - PubMed
-
- Maguire AM et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology 126, 1273–1285 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

