Discovery and validation of a personalized risk predictor for incident tuberculosis in low transmission settings
- PMID: 33077958
- PMCID: PMC7614810
- DOI: 10.1038/s41591-020-1076-0
Discovery and validation of a personalized risk predictor for incident tuberculosis in low transmission settings
Abstract
The risk of tuberculosis (TB) is variable among individuals with latent Mycobacterium tuberculosis infection (LTBI), but validated estimates of personalized risk are lacking. In pooled data from 18 systematically identified cohort studies from 20 countries, including 80,468 individuals tested for LTBI, 5-year cumulative incident TB risk among people with untreated LTBI was 15.6% (95% confidence interval (CI), 8.0-29.2%) among child contacts, 4.8% (95% CI, 3.0-7.7%) among adult contacts, 5.0% (95% CI, 1.6-14.5%) among migrants and 4.8% (95% CI, 1.5-14.3%) among immunocompromised groups. We confirmed highly variable estimates within risk groups, necessitating an individualized approach to risk stratification. Therefore, we developed a personalized risk predictor for incident TB (PERISKOPE-TB) that combines a quantitative measure of T cell sensitization and clinical covariates. Internal-external cross-validation of the model demonstrated a random effects meta-analysis C-statistic of 0.88 (95% CI, 0.82-0.93) for incident TB. In decision curve analysis, the model demonstrated clinical utility for targeting preventative treatment, compared to treating all, or no, people with LTBI. We challenge the current crude approach to TB risk estimation among people with LTBI in favor of our evidence-based and patient-centered method, in settings aiming for pre-elimination worldwide.
Conflict of interest statement
J.S.D.’s institution receives investigator-initiated research grants and consultancy income from Gilead Sciences, AbbVie, Bristol Myers Squibb and Merck. The Burnet Institute receives funding from the Victorian Government Operational Infrastructure Fund. C.L. reports honoraria from Chiesi, Gilead, Insmed, Janssen, Lucane, Novartis, Oxoid, Berlin Chemie (for participation at sponsored symposia) and Oxford Immunotec (to attend a scientific advisory board meeting), all outside of the submitted work. M.S. reports receipt of test kits free of charge from Qiagen and from Oxford Immunotec for investigator-initiated research projects. I.A. reports receiving test kits free of charge from Qiagen for an investigator-initiated research project. C.E. reports receiving test kits free of charge from Qiagen for investigator-initiated research projects outside of the submitted work. The authors declare no other conflicts of interest.
Figures

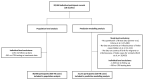
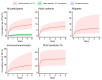



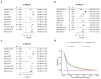
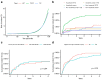




References
-
- World Health Organization. Global Tuberculosis Report. 2019. https://www.who.int/tb/publications/global_report/en/
-
- World Health Organization. The End TB Strategy. 2015. http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1 .
-
- Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127–2135. - PubMed
-
- Mack U, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956–973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

