Rapid response flow cytometric assay for the detection of antibody responses to SARS-CoV-2
- PMID: 33078221
- PMCID: PMC7572153
- DOI: 10.1007/s10096-020-04072-7
Rapid response flow cytometric assay for the detection of antibody responses to SARS-CoV-2
Abstract
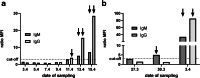
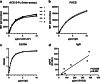
SARS-CoV-2 has emerged as a previously unknown zoonotic coronavirus that spread worldwide causing a serious pandemic. While reliable nucleic acid-based diagnostic assays were rapidly available, only a limited number of validated serological assays were available in the early phase of the pandemic. Here, we evaluated a novel flow cytometric approach to assess spike-specific antibody responses.HEK 293T cells expressing SARS-CoV-2 spike protein in its natural confirmation on the surface were used to detect specific IgG and IgM antibody responses in patient sera by flow cytometry. A soluble angiotensin-converting-enzyme 2 (ACE-2) variant was developed as external standard to quantify spike-specific antibody responses on different assay platforms. Analyses of 201 pre-COVID-19 sera proved a high assay specificity in comparison to commercially available CLIA and ELISA systems, while also revealing the highest sensitivity in specimens from PCR-confirmed SARS-CoV-2-infected patients. The external standard allowed robust quantification of antibody responses among different assay platforms. In conclusion, our newly established flow cytometric assay allows sensitive and quantitative detection of SARS-CoV-2-specific antibodies, which can be easily adopted in different laboratories and does not rely on external supply of assay kits. The flow cytometric assay also provides a blueprint for rapid development of serological tests to other emerging viral infections.
Keywords: Antibodies; Coronavirus; Flow cytometry; SARS-CoV-2; Serology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- (2020) World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 98
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

