Black-Blood Contrast in Cardiovascular MRI
- PMID: 33078512
- PMCID: PMC9292502
- DOI: 10.1002/jmri.27399
Black-Blood Contrast in Cardiovascular MRI
Abstract
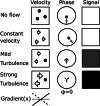
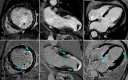
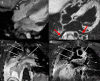
MRI is a versatile technique that offers many different options for tissue contrast, including suppressing the blood signal, so-called black-blood contrast. This contrast mechanism is extremely useful to visualize the vessel wall with high conspicuity or for characterization of tissue adjacent to the blood pool. In this review we cover the physics of black-blood contrast and different techniques to achieve blood suppression, from methods intrinsic to the imaging readout to magnetization preparation pulses that can be combined with arbitrary readouts, including flow-dependent and flow-independent techniques. We emphasize the technical challenges of black-blood contrast that can depend on flow and motion conditions, additional contrast weighting mechanisms (T1 , T2 , etc.), magnetic properties of the tissue, and spatial coverage. Finally, we describe specific implementations of black-blood contrast for different vascular beds. LEVEL OF EVIDENCE: 5 TECHNICAL EFFICACY STAGE: 5.
Keywords: black-blood contrast; blood signal suppression; vessel wall imaging.
© 2020 The Authors. Journal of Magnetic Resonance Imaging published by Wiley Periodicals LLC. on behalf of International Society for Magnetic Resonance in Medicine.
Figures








References
-
- Makowski MR, Henningsson M, Spuentrup E, et al. Characterization of coronary atherosclerosis by magnetic resonance imaging. Circulation 2013;128(11):1244‐1255. - PubMed
-
- Constable RT, Anderson AW, Zhong J, Gore JC. Factors influencing contrast in fast spin‐echo MR imaging. Magn Reson Imaging 1992;10(4):497‐511. - PubMed
-
- Mulkern RV, Melki PS, Jakab P, Higuchi N, Jolesz FA. Phase‐encode order and its effect on contrast and artifact in single‐shot RARE sequences. Med Phys 1991;18(5):1032‐1037. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

