Therapeutic Efficacy of Artemether-Lumefantrine for Uncomplicated Falciparum Malaria in Northern Zambia
- PMID: 33078701
- PMCID: PMC7695049
- DOI: 10.4269/ajtmh.20-0852
Therapeutic Efficacy of Artemether-Lumefantrine for Uncomplicated Falciparum Malaria in Northern Zambia
Abstract
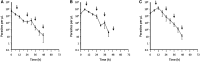
Artemether-lumefantrine (AL) is a first-line agent for uncomplicated malaria caused by Plasmodium falciparum. The WHO recommends periodic therapeutic efficacy studies of antimalarial drugs for the detection of malaria parasite drug resistance and to inform national malaria treatment policies. We conducted a therapeutic efficacy study of AL in a high malaria transmission region of northern Zambia from December 2014 to July 2015. One hundred children of ages 6 to 59 months presenting to a rural health clinic with uncomplicated falciparum malaria were admitted for treatment with AL (standard 6-dose regimen) and followed weekly for 5 weeks. Parasite counts were taken every 6 hours during treatment to assess parasite clearance. Recurrent episodes during follow-up (n = 14) were genotyped to distinguish recrudescence from reinfection and to identify drug resistance single nucleotide polymorphisms (SNPs) and multidrug resistance protein 1 (mdr1) copy number variation. Day 7 lumefantrine concentrations were measured for correspondence with posttreatment reinfection. All children who completed the parasite clearance portion of the study (n = 94) were microscopy-negative by 72 hours. The median parasite elimination half-life was 2.7 hours (interquartile range: 2.1-3.3). Genotype-corrected therapeutic efficacy was 98.8% (95% CI: 97.6-100). Purported artemisinin and lumefantrine drug resistance SNPs in atp6, 3D7_1451200, and mdr1 were detected but did not correlate with parasite recurrence, nor did day 7 lumefantrine concentrations. In summary, AL was highly effective for the treatment of uncomplicated falciparum malaria in northern Zambia during the study period. The high incidence of recurrent parasitemia was consistent with reinfection due to high, perennial malaria transmission.
Figures
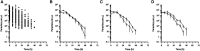

Similar articles
-
Increasing day three parasitaemia is observed after treatment of patients with artemether-lumefantrine and single dose of primaquine for uncomplicated Plasmodium falciparum malaria in Arbaminch Zuria district, Southwest Ethiopia.Malar J. 2025 Mar 18;24(1):89. doi: 10.1186/s12936-025-05337-2. Malar J. 2025. PMID: 40102850 Free PMC article.
-
Parasite clearance, cure rate, post-treatment prophylaxis and safety of standard 3-day versus an extended 6-day treatment of artemether-lumefantrine and a single low-dose primaquine for uncomplicated Plasmodium falciparum malaria in Bagamoyo district, Tanzania: a randomized controlled trial.Malar J. 2020 Jun 23;19(1):216. doi: 10.1186/s12936-020-03287-5. Malar J. 2020. PMID: 32576258 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Artemether-Lumefantrine Against Uncomplicated Falciparum Malaria Infection in Tanzania, 2022: A Single-Arm Clinical Trial.J Infect Dis. 2025 Feb 4;231(1):251-259. doi: 10.1093/infdis/jiae425. J Infect Dis. 2025. PMID: 39186698 Clinical Trial.
-
Asymptomatic recrudescence after artemether-lumefantrine treatment for uncomplicated falciparum malaria: a systematic review and meta-analysis.Malar J. 2020 Dec 9;19(1):453. doi: 10.1186/s12936-020-03520-1. Malar J. 2020. PMID: 33298080 Free PMC article.
-
The assessment of antimalarial drug efficacy in vivo.Trends Parasitol. 2022 Aug;38(8):660-672. doi: 10.1016/j.pt.2022.05.008. Epub 2022 Jun 6. Trends Parasitol. 2022. PMID: 35680541 Free PMC article. Review.
Cited by
-
Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Arba Minch Zuria District, Gamo Zone, Southwest Ethiopia.Malar J. 2024 Sep 17;23(1):282. doi: 10.1186/s12936-024-05087-7. Malar J. 2024. PMID: 39289715 Free PMC article.
-
National genomic profiling of Plasmodium falciparum antimalarial resistance in Zambian children participating in the 2018 Malaria Indicator Survey.medRxiv [Preprint]. 2024 Aug 6:2024.08.05.24311512. doi: 10.1101/2024.08.05.24311512. medRxiv. 2024. PMID: 39148823 Free PMC article. Preprint.
-
Temporal genomic analysis of Plasmodium falciparum reveals increased prevalence of mutations associated with delayed clearance following treatment with artemisinin-lumefantrine in Choma District, Southern Province, Zambia.medRxiv [Preprint]. 2024 Jun 6:2024.06.05.24308497. doi: 10.1101/2024.06.05.24308497. medRxiv. 2024. PMID: 38883763 Free PMC article. Preprint.
-
Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: A systematic review and meta-analysis.PLoS One. 2022 Mar 10;17(3):e0264339. doi: 10.1371/journal.pone.0264339. eCollection 2022. PLoS One. 2022. PMID: 35271592 Free PMC article.
-
Malaria in Refugee Children Resettled to a Holoendemic Area of Sub-Saharan Africa.Clin Infect Dis. 2023 Feb 8;76(3):e1104-e1113. doi: 10.1093/cid/ciac417. Clin Infect Dis. 2023. PMID: 35640824 Free PMC article.
References
-
- World Health Organization , 2009. Methods of Surveillance of Antimalarial Drug Efficacy. Geneva, Switzerland: WHO Global Malaria Programme.
-
- World Health Organization , 2019. World Malaria Report. Geneva, Switzerland: WHO Global Malaria Programme.
-
- Steketee RW, Sipilanyambe N, Chimumbwa J, Banda JJ, Mohamed A, Miller J, Basu S, Miti SK, Campbell CC, 2008. National malaria control and scaling up for impact: the Zambia experience through 2006. Am J Trop Med Hyg 79: 45–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

