Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209)
- PMID: 33078978
- PMCID: PMC7676887
- DOI: 10.1200/JCO.20.01076
Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209)
Abstract
Purpose: Limitations of the paclitaxel-doxorubicin-cisplatin (TAP) regimen in the treatment of endometrial cancer include tolerability and cumbersome scheduling. The Gynecologic Oncology Group studied carboplatin plus paclitaxel (TC) as a noninferior alternative to TAP.
Methods: GOG0209 was a phase III, randomized, noninferiority, open-label trial. Inclusion criteria were stage III, stage IV, and recurrent endometrial cancers; performance status 0-2; and adequate renal, hepatic, and marrow function. Prior radiotherapy and/or hormonal therapy were permitted, but chemotherapy, including radiosensitization, was not. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony-stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles. The primary endpoint was overall survival (OS; modified intention to treat). Progression-free survival (PFS), health-related quality of life (HRQoL), and toxicity were secondary endpoints.
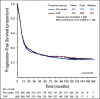
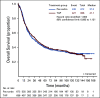
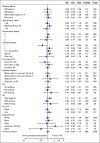
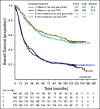
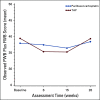
Results: From 2003 to 2009, 1,381 women were enrolled. Noninferiority of TC to TAP was concluded for OS (median, 37 v 41 months, respectively; hazard ratio [HR], 1.002; 90% CI, 0.9 to 1.12), and PFS (median, 13 v 14 months; HR, 1.032; 90% CI, 0.93 to 1.15). Neutropenic fever was reported in 7% of patients receiving TAP and 6% of those receiving TC. Grade > 2 sensory neuropathy was recorded in 26% of patients receiving TAP and 20% receiving TC (P = .40). More grade ≥ 3 thrombocytopenia (23% v 12%), vomiting (7% v 4%), diarrhea (6% v 2%), and metabolic (14% v 8%) toxicities were reported with TAP. Neutropenia (52% v 80%) was more common with TC. Small HRQoL differences favored TC.
Conclusion: With demonstrated noninferiority to TAP, TC is the global first-line standard for advanced endometrial cancer.
Trial registration: ClinicalTrials.gov NCT00063999.
Figures
References
-
- Miller DS, Randall ME, Filiaci V. Progress in endometrial cancer: Contributions of the former Gynecologic Oncology Group. Gynecol Oncol. 2020;157:312–322. - PubMed
-
- Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A gynecologic oncology group study. J Clin Oncol. 2004;22:3902–3908. - PubMed
-
- van Wijk FH, Aapro MS, Bolis G, et al. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: Definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003;14:441–448. - PubMed
-
- Gallion HH, Brunetto VL, Cibull M, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3808–3813. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

