Intra-articular Corticosteroid Injections for the Treatment of Hip and Knee Osteoarthritis-related Pain: Considerations and Controversies with a Focus on Imaging- Radiology Scientific Expert Panel
- PMID: 33079000
- PMCID: PMC7706887
- DOI: 10.1148/radiol.2020200771
Intra-articular Corticosteroid Injections for the Treatment of Hip and Knee Osteoarthritis-related Pain: Considerations and Controversies with a Focus on Imaging- Radiology Scientific Expert Panel
Abstract
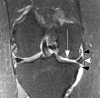
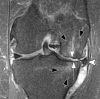
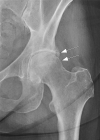
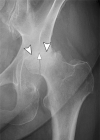
Current management of osteoarthritis (OA) is primarily focused on symptom control. Intra-articular corticosteroid (IACS) injections are often used for pain management of hip and knee OA in patients who have not responded to oral or topical analgesics. Recent case series suggested that negative structural outcomes including accelerated OA progression, subchondral insufficiency fracture, complications of pre-existing osteonecrosis, and rapid joint destruction (including bone loss) may be observed in patients who received IACS injections. This expert panel report reviews the current understanding of pain in OA, summarizes current international guidelines regarding indications for IACS injection, and considers preinterventional safety measures, including imaging. Potential profiles of those who would likely benefit from IACS injection and a suggestion for an updated patient consent form are presented. As of today, there is no established recommendation or consensus regarding imaging, clinical, or laboratory markers before an IACS injection to screen for OA-related imaging abnormalities. Repeating radiographs before each subsequent IACS injection remains controversial. The true cause and natural history of these complications are unclear and require further study. To determine the cause and natural history, large prospective studies evaluating the risk of accelerated OA or joint destruction after IACS injections are needed. However, given the relatively rare incidence of these adverse outcomes, any clinical trial would be challenging in design and a large number of patients would need to be included.
© RSNA, 2020.
Figures



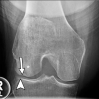

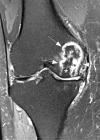
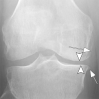

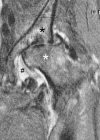




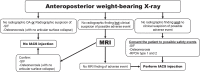
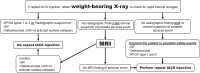
References
-
- Turkiewicz A, Petersson IF, Björk J, et al. . Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthritis Cartilage 2014;22(11):1826–1832. - PubMed
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545–1602 [Published correction appears in Lancet 2017;389(10064):e1.]. - PMC - PubMed
-
- U.S. Department of Health and Human Services, Food and Drug Administration . Osteoarthritis: Structural Endpoints for the Development of Drugs. Devices, and Biological Products for Treatment Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Published 2018. Accessed September 24, 2020.
-
- Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol 2013;9(4):236–251. - PubMed
-
- Guermazi A, Roemer FW, Crema MD, Englund M, Hayashi D. Imaging of non-osteochondral tissues in osteoarthritis. Osteoarthritis Cartilage 2014;22(10):1590–1605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

