Investigating the trade-off between folding and function in a multidomain Y-family DNA polymerase
- PMID: 33079059
- PMCID: PMC7641590
- DOI: 10.7554/eLife.60434
Investigating the trade-off between folding and function in a multidomain Y-family DNA polymerase
Abstract
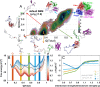
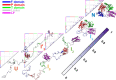
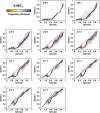
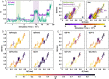
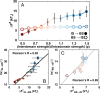
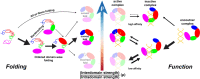
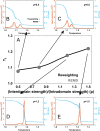
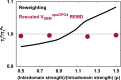
The way in which multidomain proteins fold has been a puzzling question for decades. Until now, the mechanisms and functions of domain interactions involved in multidomain protein folding have been obscure. Here, we develop structure-based models to investigate the folding and DNA-binding processes of the multidomain Y-family DNA polymerase IV (DPO4). We uncover shifts in the folding mechanism among ordered domain-wise folding, backtracking folding, and cooperative folding, modulated by interdomain interactions. These lead to 'U-shaped' DPO4 folding kinetics. We characterize the effects of interdomain flexibility on the promotion of DPO4-DNA (un)binding, which probably contributes to the ability of DPO4 to bypass DNA lesions, which is a known biological role of Y-family polymerases. We suggest that the native topology of DPO4 leads to a trade-off between fast, stable folding and tight functional DNA binding. Our approach provides an effective way to quantitatively correlate the roles of protein interactions in conformational dynamics at the multidomain level.
Keywords: coarse-grained model; computational biology; conformational dynamics; energy landscape; molecular biophysics; none; protein folding; protein-DNA recognition; structural biology; systems biology.
© 2020, Chu et al.
Conflict of interest statement
XC, ZS, JW No competing interests declared
Figures














Similar articles
-
Effects of electrostatic interactions on global folding and local conformational dynamics of a multidomain Y-family DNA polymerase.Phys Chem Chem Phys. 2021 Sep 29;23(37):20841-20847. doi: 10.1039/d1cp02832d. Phys Chem Chem Phys. 2021. PMID: 34533560
-
Confinement and Crowding Effects on Folding of a Multidomain Y-Family DNA Polymerase.J Chem Theory Comput. 2020 Feb 11;16(2):1319-1332. doi: 10.1021/acs.jctc.9b01146. Epub 2020 Jan 30. J Chem Theory Comput. 2020. PMID: 31972079 Free PMC article.
-
Multidomain protein solves the folding problem by multifunnel combined landscape: theoretical investigation of a Y-family DNA polymerase.J Am Chem Soc. 2012 Aug 22;134(33):13755-64. doi: 10.1021/ja3045663. Epub 2012 Aug 10. J Am Chem Soc. 2012. PMID: 22827444
-
Structure-based interpretation of missense mutations in Y-family DNA polymerases and their implications for polymerase function and lesion bypass.DNA Repair (Amst). 2002 May 30;1(5):343-58. doi: 10.1016/s1568-7864(02)00019-8. DNA Repair (Amst). 2002. PMID: 12509239 Review.
-
Protein folding simulations: from coarse-grained model to all-atom model.IUBMB Life. 2009 Jun;61(6):627-43. doi: 10.1002/iub.223. IUBMB Life. 2009. PMID: 19472192 Review.
Cited by
-
Investigating the Conformational Dynamics of a Y-Family DNA Polymerase during Its Folding and Binding to DNA and a Nucleotide.JACS Au. 2021 Dec 16;2(2):341-356. doi: 10.1021/jacsau.1c00368. eCollection 2022 Feb 28. JACS Au. 2021. PMID: 35252985 Free PMC article.
-
Perspectives on the landscape and flux theory for describing emergent behaviors of the biological systems.J Biol Phys. 2022 Mar;48(1):1-36. doi: 10.1007/s10867-021-09586-5. Epub 2021 Nov 25. J Biol Phys. 2022. PMID: 34822073 Free PMC article. Review.
-
Thumb-domain dynamics modulate the functional repertoire of DNA-Polymerase IV (DinB).Nucleic Acids Res. 2023 Jul 21;51(13):7036-7052. doi: 10.1093/nar/gkad490. Nucleic Acids Res. 2023. PMID: 37260088 Free PMC article.
-
Highly Dynamic Polynuclear Metal Cluster Revealed in a Single Metallothionein Molecule.Research (Wash D C). 2021 Jul 14;2021:9756945. doi: 10.34133/2021/9756945. eCollection 2021. Research (Wash D C). 2021. PMID: 34368766 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

