Factors Associated With US Adults' Likelihood of Accepting COVID-19 Vaccination
- PMID: 33079199
- PMCID: PMC7576409
- DOI: 10.1001/jamanetworkopen.2020.25594
Factors Associated With US Adults' Likelihood of Accepting COVID-19 Vaccination
Erratum in
-
Error in Results.JAMA Netw Open. 2020 Nov 2;3(11):e2030649. doi: 10.1001/jamanetworkopen.2020.30649. JAMA Netw Open. 2020. PMID: 33226426 Free PMC article. No abstract available.
Abstract
Importance: The development of a coronavirus disease 2019 (COVID-19) vaccine has progressed at unprecedented speed. Widespread public uptake of the vaccine is crucial to stem the pandemic.
Objective: To examine the factors associated with survey participants' self-reported likelihood of selecting and receiving a hypothetical COVID-19 vaccine.
Design, setting, and participants: A survey study of a nonprobability convenience sample of 2000 recruited participants including a choice-based conjoint analysis was conducted to estimate respondents' probability of choosing a vaccine and willingness to receive vaccination. Participants were asked to evaluate their willingness to receive each hypothetical vaccine individually. The survey presented respondents with 5 choice tasks. In each, participants evaluated 2 hypothetical COVID-19 vaccines and were asked whether they would choose vaccine A, vaccine B, or neither vaccine. Vaccine attributes included efficacy, protection duration, major adverse effects, minor adverse effects, US Food and Drug Administration (FDA) approval process, national origin of vaccine, and endorsement. Levels of each attribute for each vaccine were randomly assigned, and attribute order was randomized across participants. Survey data were collected on July 9, 2020.
Main outcomes and measures: Average marginal component effect sizes and marginal means were calculated to estimate the relationship between each vaccine attribute level and the probability of the respondent choosing a vaccine and self-reported willingness to receive vaccination.
Results: A total of 1971 US adults responded to the survey (median age, 43 [interquartile range, 30-58] years); 999 (51%) were women, 1432 (73%) White, 277 (14%) were Black, and 190 (10%) were Latinx. An increase in efficacy from 50% to 70% was associated with a higher probability of choosing a vaccine (coefficient, 0.07; 95% CI, 0.06-0.09), and an increase from 50% to 90% was associated with a higher probability of choosing a vaccine (coefficient, 0.16; 95% CI, 0.15-0.18). An increase in protection duration from 1 to 5 years was associated with a higher probability of choosing a vaccine (coefficient, 0.05 95% CI, 0.04-0.07). A decrease in the incidence of major adverse effects from 1 in 10 000 to 1 in 1 000 000 was associated with a higher probability of choosing a vaccine (coefficient, 0.07; 95% CI, 0.05-0.08). An FDA emergency use authorization was associated with a lower probability of choosing a vaccine (coefficient, -0.03; 95% CI, -0.04 to -0.01) compared with full FDA approval. A vaccine that originated from a non-US country was associated with a lower probability of choosing a vaccine (China: -0.13 [95% CI, -0.15 to -0.11]; UK: -0.04 [95% CI, -0.06 to -0.02]). Endorsements from the US Centers for Disease Control and Prevention (coefficient, 0.09; 95% CI, 0.07-0.11) and the World Health Organization (coefficient, 0.06; 95% CI, 0.04-0.08), compared with an endorsement from President Trump were associated with higher probabilities of choosing a vaccine. Analyses of participants' willingness to receive each vaccine when assessed individually yielded similar results. An increase in efficacy from 50% to 90% was associated with a 10% higher marginal mean willingness to receive a vaccine (from 0.51 to 0.61). A reduction in the incidence of major side effects was associated with a 4% higher marginal mean willingness to receive a vaccine (from 0.54 to 0.58). A vaccine originating in China was associated with a 10% lower willingness to receive a vaccine vs one developed in the US (from 0.60 to 0.50) Endorsements from the Centers for Disease Control and Prevention and World Health Organization were associated with increases in willingness to receive a vaccine (7% and 6%, respectively) from a baseline endorsement by President Trump (from 0.52 to 0.59 and from 0.52 to 0.58, respectively).
Conclusions and relevance: In this survey study of US adults, vaccine-related attributes and political characteristics were associated with self-reported preferences for choosing a hypothetical COVID-19 vaccine and self-reported willingness to receive vaccination. These results may help inform public health campaigns to address vaccine hesitancy when a COVID-19 vaccine becomes available.
Conflict of interest statement
Figures

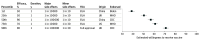
Comment in
-
Building Trust to Achieve Confidence in COVID-19 Vaccines.JAMA Netw Open. 2020 Oct 1;3(10):e2025672. doi: 10.1001/jamanetworkopen.2020.25672. JAMA Netw Open. 2020. PMID: 33079194 No abstract available.
References
-
- D’Souza G, Dowdy D What is herd immunity and how can we achieve it with COVID-19 ? Johns Hopkins Bloomberg School of Public Health Expert Insights. Published April 10, 2020. Accessed July 11, 2020. https://www.jhsph.edu/covid-19/articles/achieving-herd-immunity-with-cov...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

