Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices
- PMID: 33079460
- PMCID: PMC7681059
- DOI: 10.1111/acel.13259
Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices
Abstract
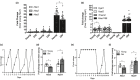
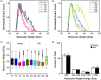
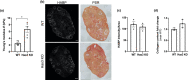
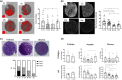
Fibrosis is a hallmark of aging tissues which often leads to altered architecture and function. The ovary is the first organ to show overt signs of aging, including increased fibrosis in the ovarian stroma. How this fibrosis affects ovarian biomechanics and the underlying mechanisms are unknown. Using instrumental indentation, we demonstrated a quantitative increase in ovarian stiffness, as evidenced by an increase in Young's modulus, when comparing ovaries from reproductively young (6-12 weeks) and old (14-17 months) mice. This ovarian stiffness was dependent on collagen because ex vivo enzyme-mediated collagen depletion in ovaries from reproductively old mice restored their collagen content and biomechanical properties to those of young controls. In addition to collagen, we also investigated the role of hyaluronan (HA) in regulating ovarian stiffness. HA is an extracellular matrix glycosaminoglycan that maintains tissue homeostasis, and its loss can change the biomechanical properties of tissues. The total HA content in the ovarian stroma decreased with age, and this was associated with increased hyaluronidase (Hyal1) and decreased hyaluronan synthase (Has3) expression. These gene expression differences were not accompanied by changes in ovarian HA molecular mass distribution. Furthermore, ovaries from mice deficient in HAS3 were stiffer compared to age-matched WT mice. Our results demonstrate that the ovary becomes stiffer with age and that both collagen and HA matrices are contributing mechanisms regulating ovarian biomechanics. Importantly, the age-associated increase in collagen and decrease in HA are conserved in the human ovary and may impact follicle development and oocyte quality.
Keywords: Biomechanics; extracellular matrix; fibrosis; hyaluronan synthase; hyaluronidase; reproduction.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Achterberg, V. F. , Buscemi, L. , Diekmann, H. , Smith‐Clerc, J. , Schwengler, H. , Meister, J.‐J. , Wenck, H. , Gallinat, S. , & Hinz, B. (2014). The nano‐scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. The Journal of Investigative Dermatology, 134(7), 1862‐1872. 10.1038/jid.2014.90 - DOI - PubMed
-
- Bai, K. J. , Spicer, A. P. , Mascarenhas, M. M. , Yu, L. , Ochoa, C. D. , Garg, H. G. , & Quinn, D. A. (2005). The role of hyaluronan synthase 3 in ventilator‐induced lung injury. American Journal of Respiratory and Critical Care Medicine, 172(1), 92‐98. 10.1164/rccm.200405-652OC - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

