Polygenic Contribution to Low-Density Lipoprotein Cholesterol Levels and Cardiovascular Risk in Monogenic Familial Hypercholesterolemia
- PMID: 33079599
- PMCID: PMC7889287
- DOI: 10.1161/CIRCGEN.120.002919
Polygenic Contribution to Low-Density Lipoprotein Cholesterol Levels and Cardiovascular Risk in Monogenic Familial Hypercholesterolemia
Abstract
Background: Familial hypercholesterolemia (FH) is a common autosomal codominant genetic disorder, which causes elevated levels of low-density lipoprotein cholesterol (LDL-C) and increased risk of premature atherosclerotic cardiovascular disease (ASCVD). Even among individuals with monogenic FH, there is substantial interindividual variability in LDL-C levels and risk of ASCVD. We assessed the influence of an LDL-C polygenic score on levels of LDL-C and risk of ASCVD for individuals with monogenic FH.
Methods: We constructed a weighted LDL-C polygenic score, composed of 28 single-nucleotide variants, for individuals with monogenic FH from the British Columbia FH (n=262); Nutrition, Metabolism and Atherosclerosis Clinic (n=552); and UK Biobank cohorts (n=306). We assessed the association between LDL-C polygenic score with LDL-C levels and ASCVD risk using linear regression and Cox-proportional hazard models, respectively. ASCVD was defined as myocardial infarction, coronary or carotid revascularization, transient ischemic attack, or stroke. The results from individual cohorts were combined in fixed-effect meta-analyses.
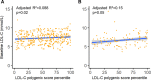
Results: Levels of LDL-C were significantly associated with LDL-C polygenic score in the Nutrition, Metabolism and Atherosclerosis Clinic cohort, UK Biobank cohort, and in the meta-analysis (β [95% CI]=0.13 [0.072-0.19] per a 20% increase in LDL-C polygenic score percentile, P<0.0001). Additionally, an elevated LDL-C polygenic score (≥80th percentile) was associated with a trend towards increased ASCVD risk in all 3 cohorts individually. This association was statistically significant in the meta-analysis (hazard ratio [95% CI]=1.48 [1.02-2.14], P=0.04).
Conclusions: Polygenic contributions to LDL-C explain some of the heterogeneity in clinical presentation and ASCVD risk for individuals with FH.
Keywords: coronary artery disease; hypercholesterolemia; lipids; lipoproteins; metabolism.
Figures



References
-
- Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the copenhagen general population study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–1394. doi: 10.1093/eurheartj/ehw028 - PubMed
-
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144 - PMC - PubMed
-
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, et al. ; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European atherosclerosis society. Eur Heart J. 2013;34:3478–390a. doi: 10.1093/eurheartj/eht273 - PMC - PubMed
-
- Berberich AJ, Hegele RA. The complex molecular genetics of familial hypercholesterolaemia. Nat Rev Cardiol. 2019;16:9–20. doi: 10.1038/s41569-018-0052-6 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

