Dietary cellulose induces anti-inflammatory immunity and transcriptional programs via maturation of the intestinal microbiota
- PMID: 33079623
- PMCID: PMC7583510
- DOI: 10.1080/19490976.2020.1829962
Dietary cellulose induces anti-inflammatory immunity and transcriptional programs via maturation of the intestinal microbiota
Abstract
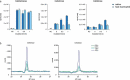
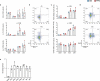
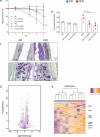
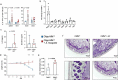
Although it is generally accepted that dietary fiber is health promoting, the underlying immunological and molecular mechanisms are not well defined, especially with respect to cellulose, the most ubiquitous dietary fiber. Here, the impact of dietary cellulose on intestinal microbiota, immune responses and gene expression in health and disease was examined. Lack of dietary cellulose disrupted the age-related diversification of the intestinal microbiota, which subsequently remained in an immature state. Interestingly, one of the most affected microbial genera was Alistipes which is equipped with enzymes to degrade cellulose. Absence of cellulose changed the microbial metabolome, skewed intestinal immune responses toward inflammation, altered the gene expression of intestinal epithelial cells and mice showed increased sensitivity to colitis induction. In contrast, mice with a defined microbiota including A. finegoldii showed enhanced colonic expression of intestinal IL-22 and Reg3γ restoring intestinal barrier function. This study supports the epidemiological observations and adds a causal explanation for the health promoting effects of the most common biopolymer on earth.
Keywords: Alistipes; Cellulose; IL-22; Reg3γ; bile acids; inflammation; insoluble fiber; microbial diversity; microbiota maturation; mucosal homeostasis.
Conflict of interest statement
No potential conflict of interest was reported by the authors
Figures



References
-
- Burkitt D, Trowell HC. Refined carbohydrate foods and disease: some implications of dietary fibre. New York: Academic Press Inc; 1975.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
